Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 17
30 January, 2019
CASE III: D12-44205-1A or B UMN VDL (JPC 4032592)
Signalment: One-month-old, intact male, white Hartley guinea pig (Cavia porcellus).
History: The guinea pig, purchased and shipped from a source colony, was placed in quarantine for an acclimation period of 7 days. The guinea pig was released from quarantine on day 7, placed on study day 9 involving an induction dose (6 intradermal injections of 0.1 ml of test article extract in normal saline, normal saline and/or Freund’s Complete Adjuvant). The guinea pig lost body condition and exhibited progressive respiratory distress over days 10 and 11. The guinea pig was found dead on day 12.
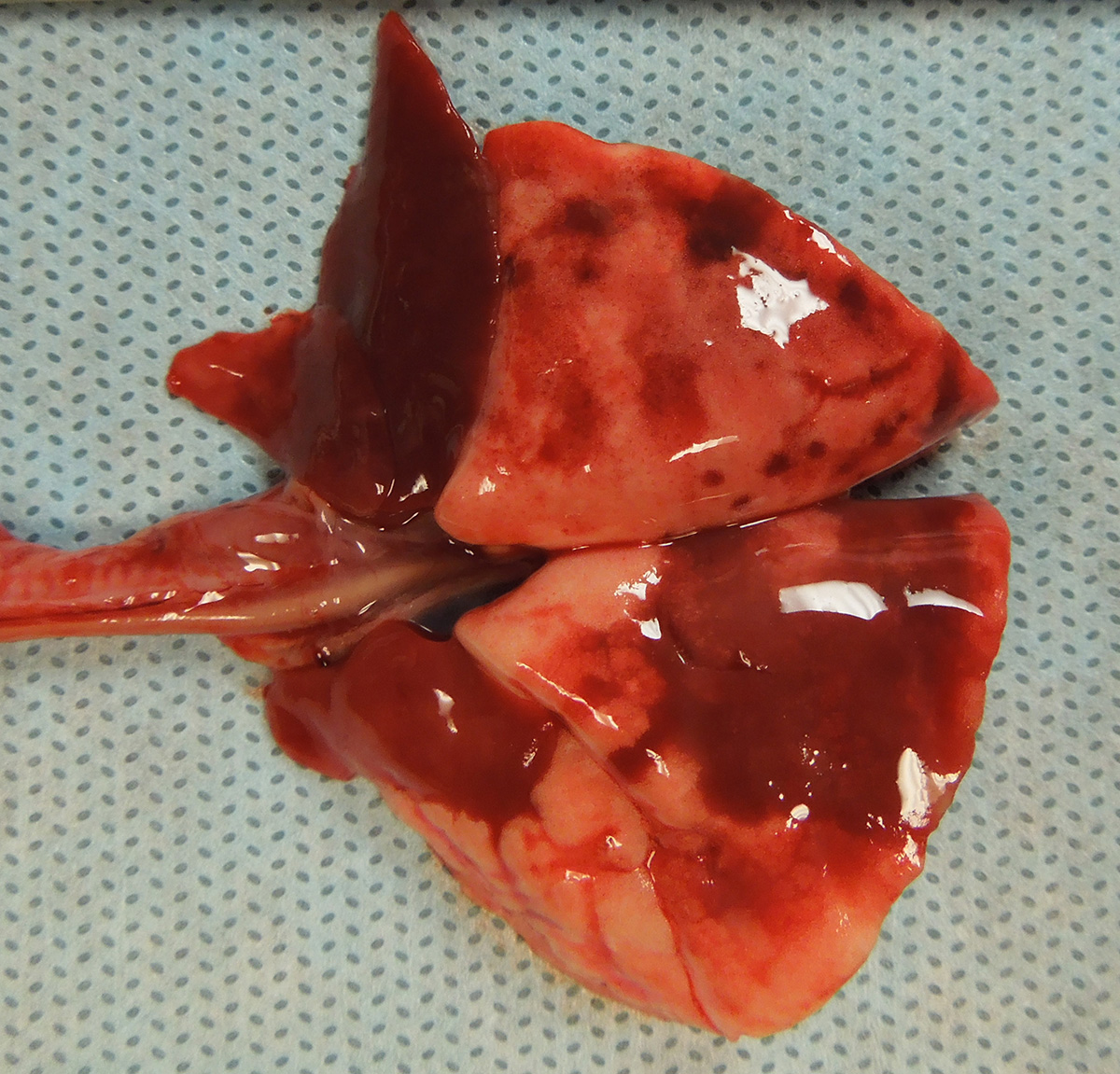
Gross Pathology: The guinea pig was in good nutritional condition with mild postmortem autolysis. The thoracic cavity contained approximately 0.5 ml of watery, red, clear fluid. The left and right cranial lung lobes were diffusely dark red, heavy and wet. Sections of the left and right cranial lung lobes sank in 10% neutral buffered formalin. The right middle and right accessory lung lobes were diffusely dark red and firm. The left and right caudal lung lobes contained multifocal to coalescing, dark red, firm, irregular, smooth, flat foci.
Laboratory results: Bacteriology: Lung - No bacteria isolated
Guinea pig adenovirus quantitative real-time polymerase chain reaction (qPCR) testing and aerobic culture of lung were performed at Charles River Research Animal Diagnostic Services. The lung sample was positive for guinea pig adenovirus via qPCR. Aerobic culture of the left cranial lung lobe yielded rare colonies of Escherichia coli.
Microscopic Description:
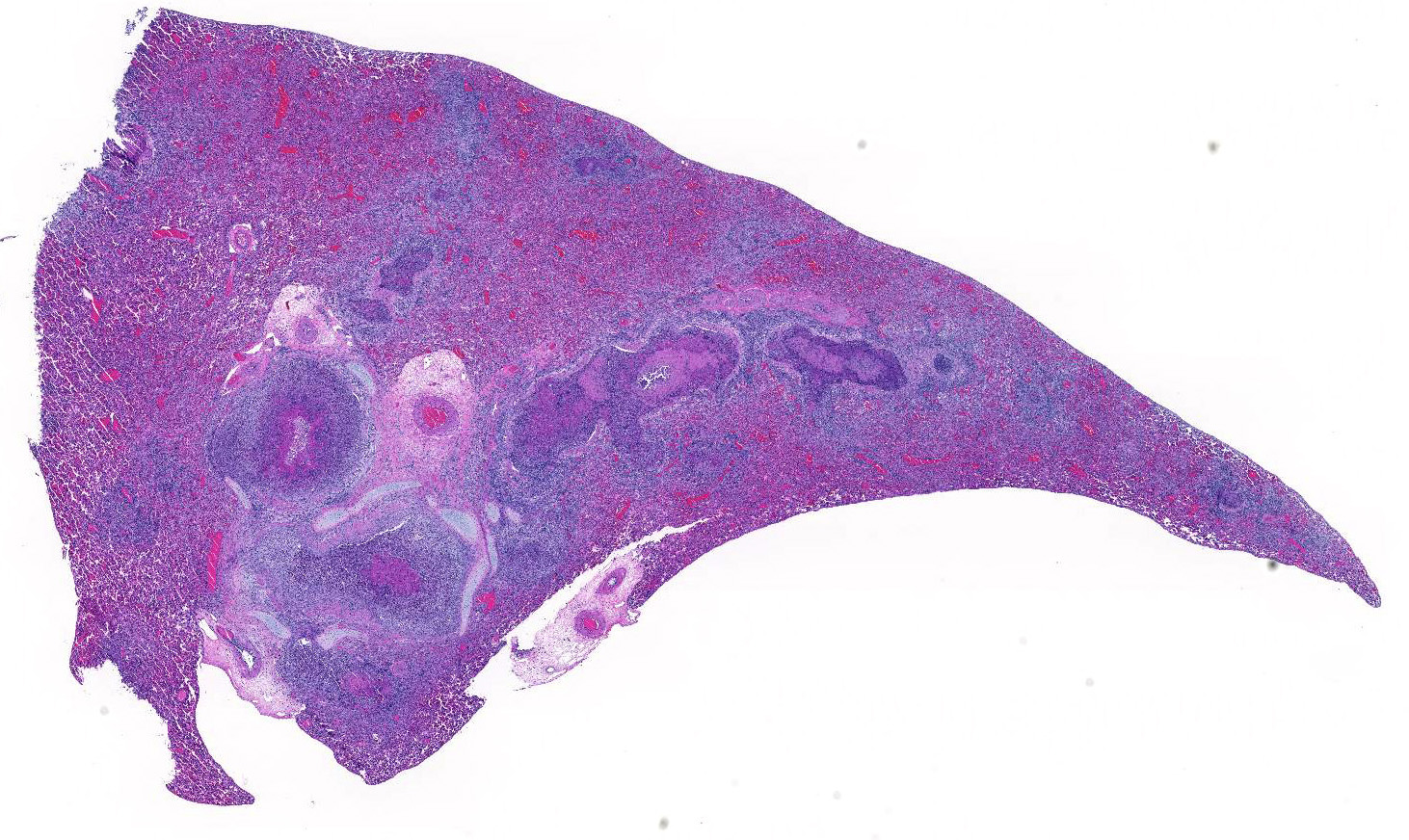
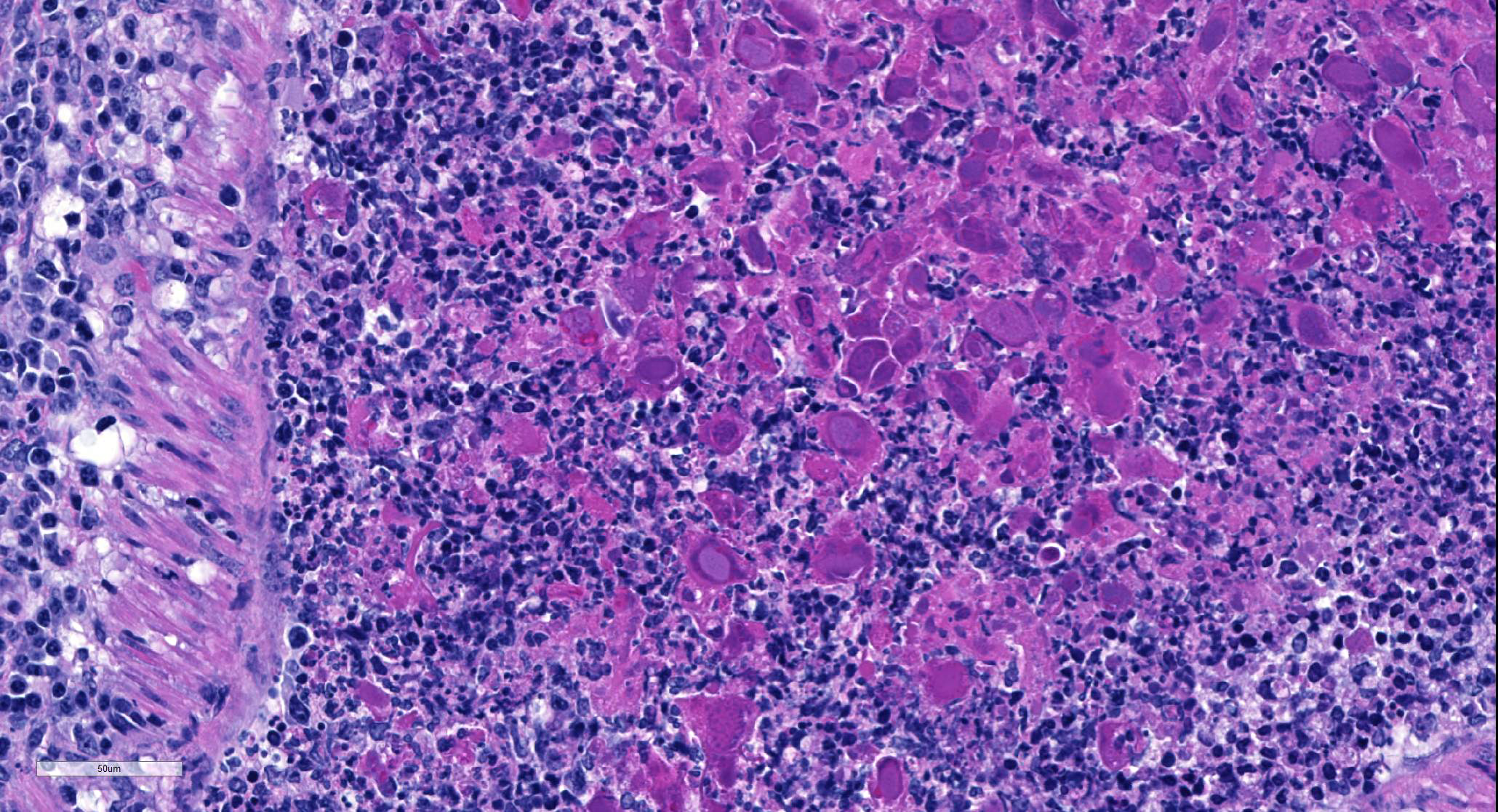
Multifocally, comprising 60 – 70% of the tissue section, within the bronchi, bronchioles and alveoli, there are infiltrates of numerous degenerate and nondegenerate neutrophils, macrophages, lymphocytes, few plasma cells and extravasated erythrocytes. The lumina of the bronchi and bronchioles are frequently mildly to moderately ectatic, containing large amounts of cellular and karyorrhectic debris, extravasated erythrocytes, fibrin and sloughed degenerate to necrotic respiratory epithelial cells that occasionally contain a single, large, 5 – 15 um diameter basophilic intranuclear inclusion body. Multifocally bronchi and bronchioles are lined by flattened, attenuated, degenerate to necrotic epithelium that frequently form epithelial syncytia or are multinucleated. Multifocally alveolar septa are mildly to moderately expanded by lymphocytes, mononuclear cells, type II pneumocytes and congested blood vessels. Multifocally interstitium is mildly to moderately expanded by neutrophils, lymphocytes and plasma cells as well as edema. The tunica media of multiple small to medium caliber vessels and muscularis of the bronchi and bronchioles exhibits vacuolar degeneration of the smooth muscle.
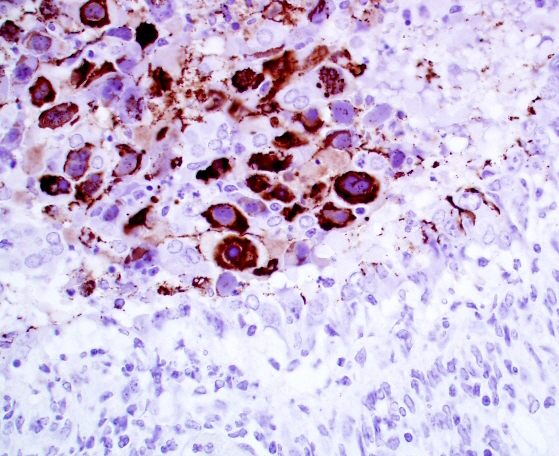
Immunohistochemistry for general adenovirus on tissue sections of lung were prepared by the Veterinary Medical Diagnostic Laboratory of the University of Missouri. Multifocally lumina of bronchioles contain numerous red to dark red, strongly immunopositive adenovirus particles, both free within the lumen admixed with cellular debris and within nuclei and cytoplasm of sloughed necrotic epithelial cells.
Electron microscopy was performed at the University of Minnesota Veterinary Diagnostic Laboratory. Bronchiolar lumina contain necrotic cells with markedly enlarged nuclei exhibiting discontinuous nuclear membranes, clumping of chromatin and lacking a clear distinction between the nucleus and the cytoplasm. The necrotic cells contain numerous loosely arranged, nonenveloped, round to icosahedral, 70 – 90 nm diameter, variably electron dense, icosahedral viral particles consistent with adenovirus.
Contributor’s Morphologic Diagnoses:
Lung, bronchopneumonia, necrosuppurative, with intraepithelial intranuclear inclusion bodies (adenovirus), multifocal, marked, subacute.
Contributor’s Comment: Adenoviruses are non-enveloped, linear double stranded DNA viruses with icosahedral symmetry. Adenoviruses infect a wide variety of animals with clinical signs ranging from subclinical to enteric or respiratory manifestations. Guinea pig adenovirus (GpAV) has been classified as a distinct serotype within the genus Mastadenovirus. GpAV has the highest level of homology with other animal mastadenoviruses (mammalian adenoviruses) and human subgroups A, C and F.5,14 Adenoviruses derived from birds (Aviadenovirus, Atadenovirus), frogs (Siadenovirus), fish (Ichtadenovirus) and some reptiles (Atadenovirus) are serologically distinct from Mastadenoviruses.9
Guinea pig adenovirus was first reported in 1981 in guinea pigs from 2 commercial breeders and a closed colony, as the cause of necrotizing bronchitis and bronchiolitis with low morbidity and high mortality.11 The adenovirus observed in this outbreak was described as viral particles of 58 – 72 nm diameter, in a hexagonal crystalline array, observed as large, basophilic, intranuclear inclusion bodies by light microscopy. Additional outbreaks of GpAV in guinea pigs were characterized by necrotizing bronchitis and bronchiolitis with similar prominent basophilic intranuclear inclusions in sloughed, necrotic epithelial cells.1 Inclusions contained typical adenoviral particles of 68 – 72 nm diameter, arranged individually or in crystalline arrays. GpAV infection was experimentally reproduced via intranasal inoculation of newborn guinea pigs and was found to be noninfectious in additional mammalian species of hamsters and rats.7,10 Subclinical infection has been reported, but the asymptomatic animals were found to have microscopic lesions of minimal bronchial epithelial necrosis with typical large basophilic intranuclear inclusions.4
Bronchopneumonia due to GpAV is typically of low morbidity with up to 100% mortality. Clinically affected animals are often young, but infections have also been reported in adult guinea pigs in breeding colonies.5,6,13 Infection of guinea pigs often presents as sudden death or death after a short period of malaise. On gross necropsy, the cranial lung lobes are usually consolidated or diffusely red, while the caudal lung lobes show multifocal consolidation. Histopathology findings are characterized by a necrotizing bronchitis and bronchiolitis with sloughing of necrotic epithelial cells into the airways, often resulting in occlusion of the affected airways with necrotic cells admixed with cell debris, leukocytes and fibrin. The necrotic epithelial cells often exhibit karyomegaly with extremely large or bizarre basophilic, round to oval, 7 – 15 um diameter, intranuclear inclusion bodies.1,7,11
Diagnosis of GpAV infection can be confirmed via immunohistochemistry, electron microscopy, serology or polymerase chain reaction. Other differentials for pneumonia in guinea pigs include parainfluenza virus, cytomegalovirus and bacteria such as Bordetella bronchiseptica, Streptococcus species, Staphylococcus species and Pseudomonas aeruginosa.13
Contributing Institution:
Veterinary Diagnostic Laboratory, University of Minnesota, www.vdl@umn.edu
JPC Diagnosis: Lung: Bronchitis and bronchiolitis, necrotizing, diffuse, severe with diffuse lymphohistiocytic peribronchiolitis and atelectasis, and numerous karyomegalic viral inclusions
JPC Comment: Adenoviruses are common viruses in a wide range of animal species, and display tropism for a wide range of tissues – endothelium (dog, deer cattle), liver (chicken, primates), and in immunosuppressed animals (and humans) , a vast array of cell types may be targeted.
A number of pneumotropic adenoviruses may result in a similar clinical and histologic picture, although immunosuppression may be required for lesion development. Canine adenovirus-2 may result in pulmonary damage in (usually immunosuppressed, often by canine distemper virus) individuals. In experimental infections in young beagles, bronchiolitis obliterans was seen 3-4 weeks following infection2 (although long-term followup was not conducted to see if the lesions resolved.) Bovine adenovirus-3 will also result in a severe necrotizing bronchiolitis with viral intranuclear inclusions in calves administered dexamethasone.12
Pneumotropic adenoviral infections are well-known in primate species. In 1953 adenoviruses were first isolated by Rowe who was studying the growth of poliovirus in adenoidal tissue. Today, over 60 human adenovirus serotypes have been identified. Disease in humans varies with age, immune status and population characteristics. Adenoviral pneumonia associated with serotypes 3,7,14, 21, and 55 have been associated with potentially fatal outcomes, and infants, immunosuppressed individuals, and the elderly are especially hard hit. Extrapulmonary complications include meningoencephalitis, hepatitis myocarditis, nephritis, neutropenia, and DIC.8
An outbreak in a national primate center in 2009 in a closed colony of titi monkeys (Callicebus sp.). 23 of 65 developed upper respiratory symptoms which progressed to fulminant pneumonia and hepatitis; 19 of these animals died. A novel, highly divergent adenovirus (TMAdV) was isolated, sharing only <57% nucleotide identity with other known adenoviruses. The virus was also isolated from a researcher who demonstrated respiratory symptoms for 4 weeks, and a close family member who had never been to the primate colony.15
In 1997, an outbreak of adenoviral pneumonia resulted in respiratory illness in 9 infant olive baboons, with two fatalities. Six untypeable adenoviruses were isolated from these animals during the course of the illness; one novel adenovirus (BaAdV-3) was determined to the cause of the disease.3
Conference attendees noted the presence of abundant viral antigen within the cytoplasm of epithelial cells within the necrotic bronchioles, with relative sparing of nuclei containing karyomegalic inclusions in the image of the immunohistochemical stain provided by the contributor (Fig 2-4). The significance of this change was not apparent.
References:
- Brennecke LH, Dreier TM, Stokes WS. Naturally occurring virus-associated respiratory disease in two guinea pigs. 1983;20:488-491.
- Castleman WL. Bronchiolitis obliterans and pneumonia induced in young dogs by experimental adenovirus infection. Am J Pathol 1985; 119(3):495-504.
- Chiu CY, Yagi S, Lu X, Yu G, Chen EC, Liu M, Dick EJ, Carey KD, Erd DD, Leland MM, Patterson JL. A novel adenovirus species associated with an acute respiratory outbreak in a baboon colony and evidence of coincident human infection. mBio 2013; 4(2):e00084-13.
- Crippa L, Giusti AM, Sironi G, et al. Asymptomatic adenoviral respiratory tract infection in guinea pigs. 1997:47(2):197-199.
- Feldman SH, Sikes R, Eckhoff G. Comparison of the deduced amino acid sequence of guinea pig adenovirus hexon protein with that of other Mastadenoviruses. Comp Med. 2001;51(2):120-126.
- Harris IE, Portas BH, Goydich W. Adenoviral bronchopneumonia of guinea pigs. Aust Vet Journal. 1985;62(9):317-318.
- Kaup, FJ, Naumann S, I Kunstyr, et al. Experimental viral pneumonia in guinea pigs: An ultrastructural study. 1984;21:521-527.
- Khanal S, Ghimire P, Dhamoon AS. The repertoire of adenovirus in human disease: the innocuous to the deadly. Biomedicines 2018; 6:30 doi: 10.3390/biomedicines6010030
- King, AMQ, Adams MJ, Carstens EB, et al. Virus Taxonomy. London, UK: International Committee on Taxonomy of Viruses, Elsevier Inc; 2012.
- Kunstyr I, Maess J, Naumann S, et al. Adenovirus pneumonia in guinea pigs: an experimental reproduction of the disease. 1984;18:55-60.
- Naumann S, Kunstyr I, Langer I, et al. Lethal pneumonia in guinea pigs associated with a virus. Lab Animals. 1981;15:235-242.
- Narita M, Yamada M, Tsuboi T, Kawashima. Bovine adenovirus type 3 pneumonia in dexamethoasone-treated calves. Vet Pathol 40:128-135.
- Percy DH, Barthold SW. Pathology of Laboratory Rodents and Rabbits, Third Edition. Ames, Iowa: Blackwell Publishing Professional; 2007.
- Pring-Akerblom P, Blazek K, Schramlova J, et al. Polymerase chain reaction for detection of guinea pig adenovirus. J Vet Diagn Invest. 1997;9:232-236.
- Yu G, Yagi S, Carrion R, Chen EC, Liu M, Brasky KM, Lanford RD, Kelly KR, Bales KL, Schnurr DP, Canfield DR, Patterson JG, Chiu CY. Experimental cross-species infection of common marmosets by titi monkey adenovirus. PLoS One 2013; 8(7):e68558