Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 6
October 3, 2018
CASE I: 21608 (JPC 4089879-00).
Signalment: 22 week old female Crl:WI(Han) rat (Rattus norvegicus)
History: Tissues were routinely collected from this control female rat at the terminal sacrifice of a 13 week oral gavage study of a drug candidate. No clinical observations were noted prior to sacrifice.
Gross Pathology: No macroscopic observations were noted.
Laboratory results: None given.
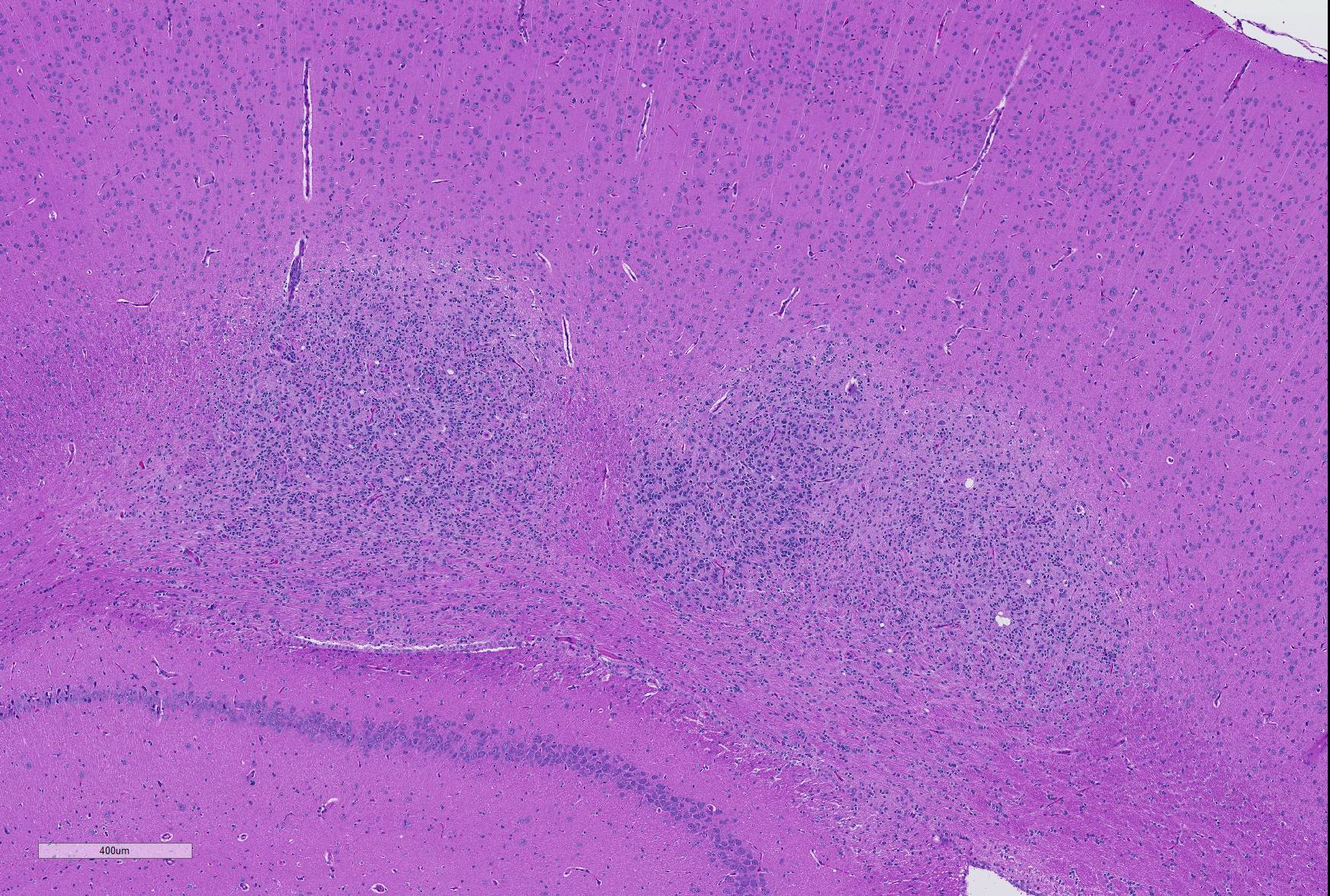
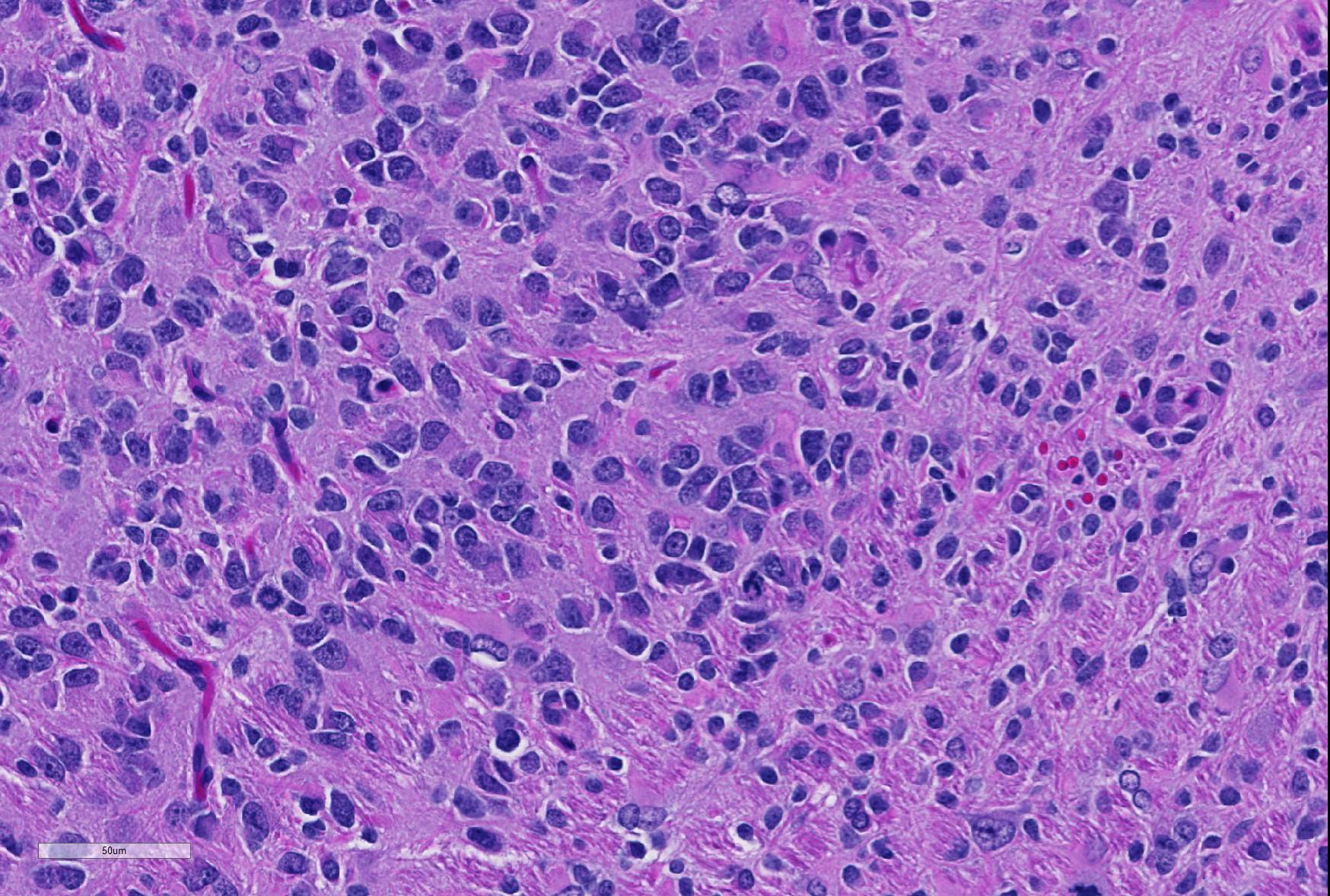
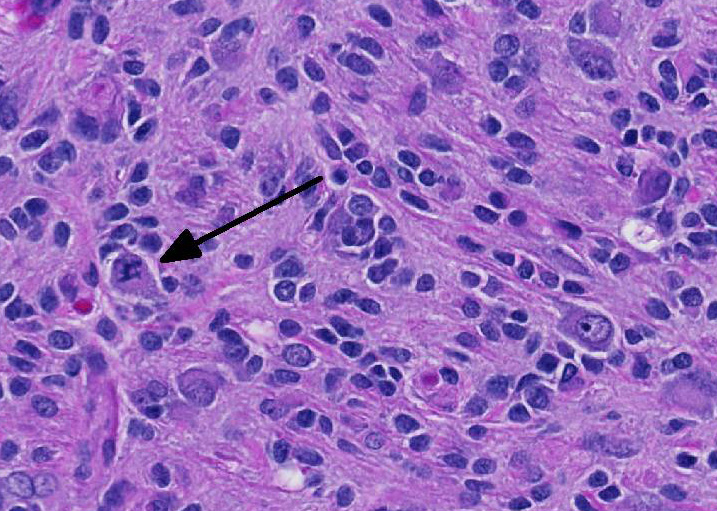
Microscopic Description: Brain: In the cerebrum, subcortical white matter in one hemisphere is focally replaced by a poorly demarcated mass composed of densely packed round to fusiform cells. These cells are often arranged in small palisades, rarely form perivascular whorls, and have indistinct cytoplasmic margins, scant to abundant abundant pale amphophilic fibrillar cytoplasm, and round to oval euchromatic nuclei that often contain one or two nucleoli. There is marked anisokaryosis and there are rare mitotic figures. Interspersed among these neoplastic cells are cells with large nuclei and abundant eosinophilic cytoplasm (interpreted as gemistocytic astrocytes).
Contributor’s Morphologic Diagnoses: Glioma, brain.
Contributor’s Comment: Glial neoplasms are the most commonly occurring spontaneous brain neoplasms in Sprague Dawley (SD) rats, with a reported incidence of approximately 1.5 – 2.0% in control rats in carcinogenicity studies, and are generally more common in males. Most of these tumors have been classified as malignant astrocytomas.1,6 Although rarely fatal during the first year of life, spontaneous glial neoplasms have been reported in SD rats as young as 21 or 32 weeks of age.3,8
Recent immunohistochemical studies of rat glial neoplasms have demonstrated that the vast majority of spontaneous and chemically induced tumors traditionally termed “astrocytomas” do not express detectable glial fibrillary acidic protein (GFAP) or other astrocyte markers,2,4,5 although GFAP positive neoplastic astrocytes have been demonstrated in oligodendrogliomas and mixed gliomas of rats.7 In contrast, most rat “astrocytomas” are immunopositive for markers of microglia and monocytes/macrophages such as ionized calcium-binding adapter molecule-1 (Iba-1) and bind the lectin Ricinus communis agglutinin type 1 (RCA-1).2,4,5 Based on these studies, the majority of rat “astrocytomas” are more appropriately termed malignant microglial tumors. It has been recommended that these tumors be diagnosed as “gliomas” in routine toxicology or carcinogenicity studies, and that more specific diagnosis as to cell of origin should be based on immunohistochemical findings.2
Contributing Institution:
Covance Laboratories, Inc, Madison, Wisconsin, USA.http://www.covance.com/industry-solutions/drug-development/services/safety-assessment/nonclinical-pathology-services.html
JPC Diagnosis: Cerebrum: Glioma.
JPC Comment: As recently as the early 2000’s, texts referred to primary tumors of the central nervous system in rats as “uncommon” neoplasms.2 This somewhat questionable statement may have resulted from the fact that 1) many of these tumors are not grossly visible, 2) brain sampling protocols generally only require three coronal sections of grossly normal brains, and 3) the relative resistance of post-natal rats to chemical induction of tumors in the central nervous system. Most brain cancer models have traditionally required intrauterine exposure at critical stages of development to carcinogenic substances. 2
In the last decade, a growing body of literature, improved sampling techniques as promoted by the Society for Toxicologic Pathologists, and the publication of historical control databases has shed more light on CNS tumors in rats.3 In general, the incidence of spontaneous CNS tumors is higher than in mice and spontaneous tumors are found more frequently in males than in females. Incidence does not differ significantly between strains. Tumors of with glial and meningeal differentiation are more commonly seen than those with neuronal or primitive neuroepithelial differentiation.3
Glial cell tumors are believed to arise from neoplastic radial glial cells (RGCs). 3 These neoplasms may move within the CSF into the spinal cord but metastasis outside the nervous system is has not yet been reported. For this reason, the terms “benign” and “malignant” have largely been replaced in the toxicologic pathology community with the terms “low-grade” and “high-grade”.3
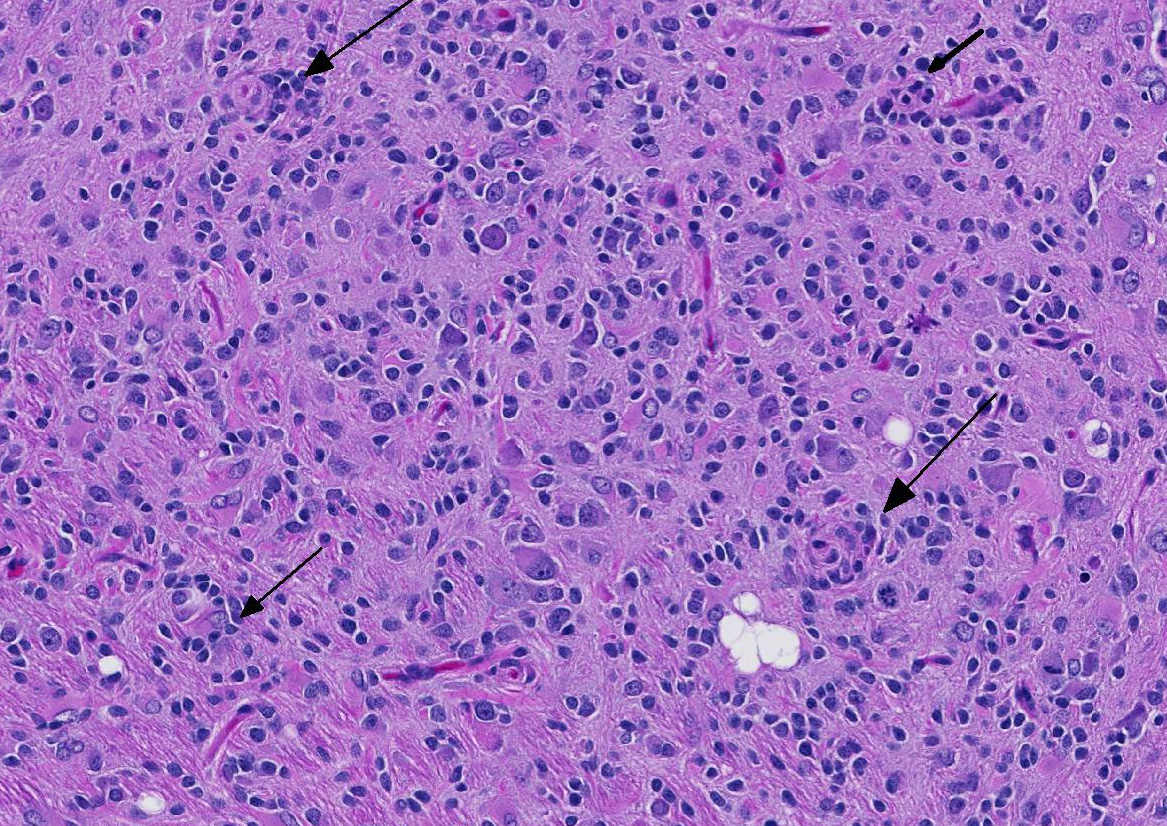
Before the advent of immunohistochemistry and other advanced diagnostics that allowed for more precise identification of the cell of origin, most glial tumors were identified as “glioma”.2 Today, immunohistochemical protocols are allowing pathologist to sort glial tumors into more specific categories with precision. Immunohistochemical and lectin profiles exist for astrocytomas and oligodendrogliomas. The term “glioma” is largely reserved for mixed tumors in which cells fitting the morphology and the immunohistochemical and lectin profiles of both astrocytoma and oligodendroglioma are present in the neoplasm. These neoplasms may be either low- or high grade tumors. High-grade gliomas are characterized by infiltration into multiple areas of the brain, cellular atypia and pleomorphism, necrosis, and rarely giant cell formation (most likely of astrocytic origin). If these changes are present and each glial cell provides at least 20% of the neoplasm, the tumor is diagnosed as a high-grade mixed glioma. Interestingly, experimental studies have indicated that gliomas an adult rats are initially composed of either differentiated astrocytes or oligodendrocytes. As these neoplasms increase in size, cellular composition becomes mixed and anaplastic over time.3 These high-grade mixed gliomas share some histologic features with the so-called glioblastoma multiforme of humans.3
The conference moderator commented on the heterogeneous nature of the neoplastic cells, with cells resembling both astrocytes and oligodendrocytes in the neoplasm, as well as accompanying activated microglia and reactive astrocytes, ultimately forming a complicated mix of morphologies. The JPC diagnosis of glioma in this case is largely based on the HE appearance.
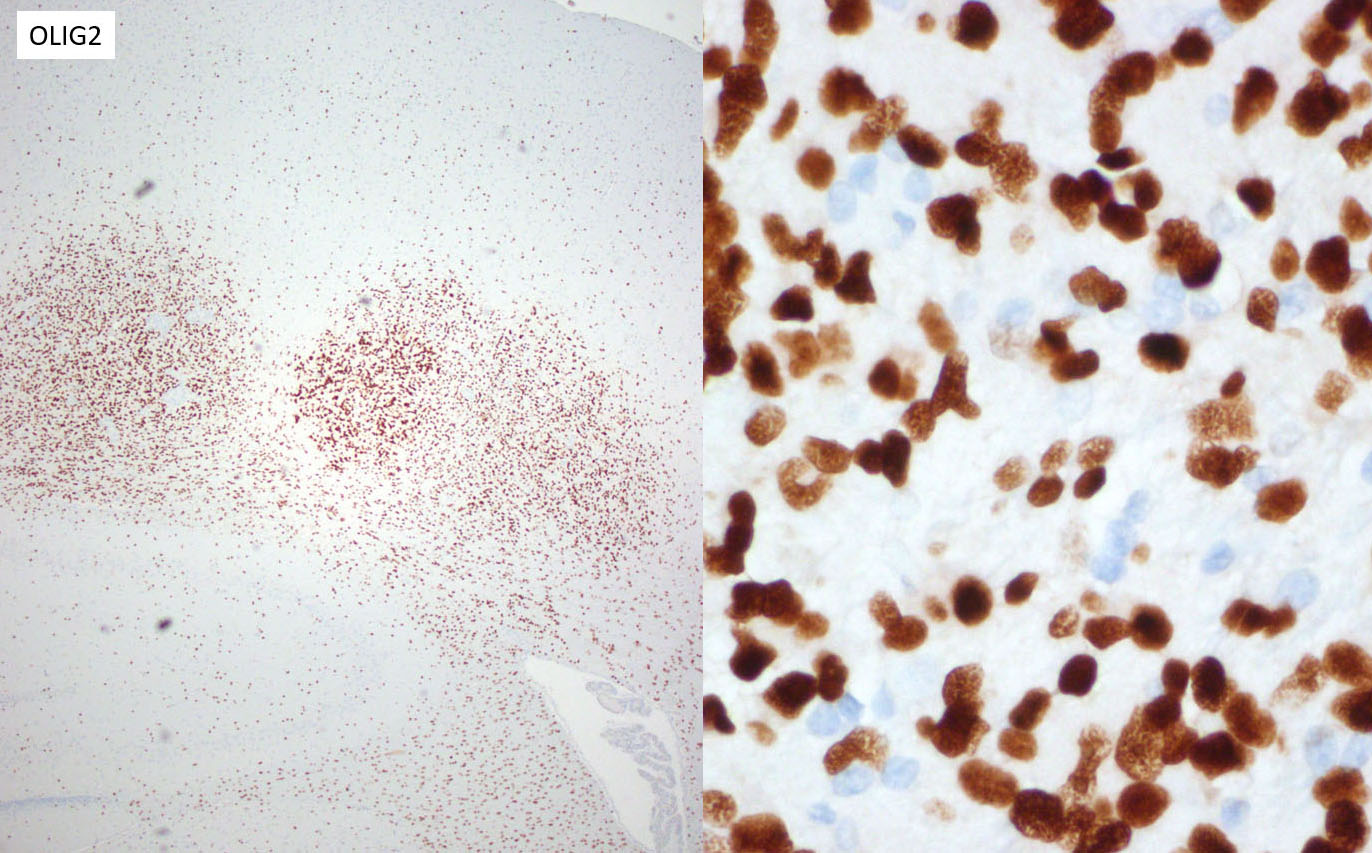
The majority of neoplastic cells were strongly immunopositive for an OLIG2 stain run at the JPC and is consistent with the diagnosis of glioma. (OLIG2, counterintuitively to its name, will stain both oligodendrocytes and astrocytes. ) While there was extensive staining with the JPC-run GFAP and Iba1 within the neoplasm, the moderator believes that this reflects reactive astrocytes and infiltrating microglia respectively, and the staining excluded neoplastic cells. Based on this additional data, the moderator believes that a diagnosis of glioma is appropriate for the HE appearance of this neoplasm, and that the immunohistochemical findings are consistent with a primitive glial tumor, but does not enable a more specific diagnosis.
References:
- Bertrand L, Mukaratirwa S, Bradley A. Incidence of spontaneous central nervous system tumors in CD-1 mice and Sprague-Dawley, Han-Wistar, and Wistar Rats used in carcinogenicity studies. Toxicol Pathol. 2014;42:1168-1173.
- Elmore SA, Farman CA, Hailey JR, et al. Proceedings of the 2015 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2016;44:502-35.
- Ikezaki S, Takagi M, Tamura K. Natural occurrence of neoplastic lesions in young Sprague-Dawley rats. J Toxicol Pathol. 2011;24:37-40.
- Kolenda-Roberts HM, Harris N, Singletary E, Hardisty JF. Immunohistochemical characterization of spontaneous and acrylonitrile-induced brain tumors in the rat. Toxicol Pathol. 2013;41:98-108.
- Nagatani M, Ando R, Yamakawa S, Saito T, Tamura K. Histological and immunohistochemical studies on spontaneous rat astrocytomas and malignant reticulosis. Toxicol Pathol. 2009;37:599-605.
- Nagatani M, Kudo K, Yamakawa S. Occurrence of spontaneous tumors in the central nervous system (CNS) of F344 and SD rats. J Toxicol Pathol. 2013;26:263-273.
- Nagatani M, Yamakawa S, Saito T, et al. GFAP-positive neoplastic astrocytes in spontaneous oligodendrogliomas and mixed gliomas in rats. Toxicol Pathol. 2013;41:653-661.
- Son W-C, Gopinath C. Early occurrence of spontaneous tumors in CD-1 mice and Sprague-Dawley rats. Toxicol Pathol. 2004;32:371-374.