Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 21
24 March 2019
CASE IV PA 16-5448 (JPC 4101316)
Signalment: Adult, female, Sussex chicken (Gallus gallus domesticus)
History: This adult female Sussex chicken was submitted along with two other hens; all with a reported history of lethargy, equilibrium imbalance, and watery stool.
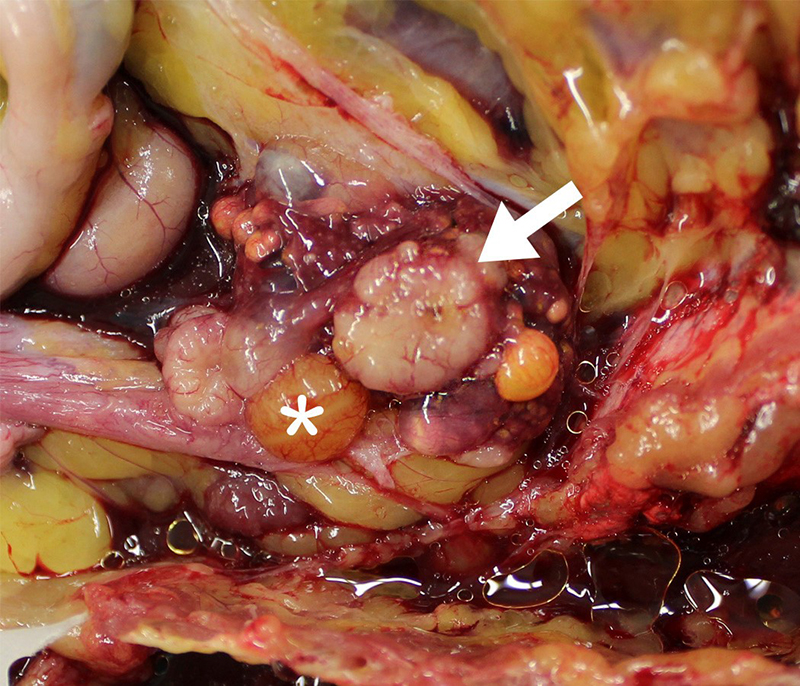
Gross Pathology: At necropsy, the ovary was smaller than expected due to lack of large follicles (the largest was 1.0 cm in diameter. There were two gray/white, solid, disc-like structures, of approximately 0.9-1.2 cm in diameter x 0.3 cm deep on the ventral aspect of the ovary The oviduct was active but underdeveloped. The left leg was swollen distal to the hock and was approximately 2-3 times the thickness of the right leg. The subcutis was expanded, wet, gelatinous and light grey to light green.
Laboratory results: A swab from the abnormal subcutaneous tissue from the left leg was cultured aerobically and yielded a few Escherichia coli, a few Staphylococcus sp., and a few Streptococcus sp.
Microscopic Description:
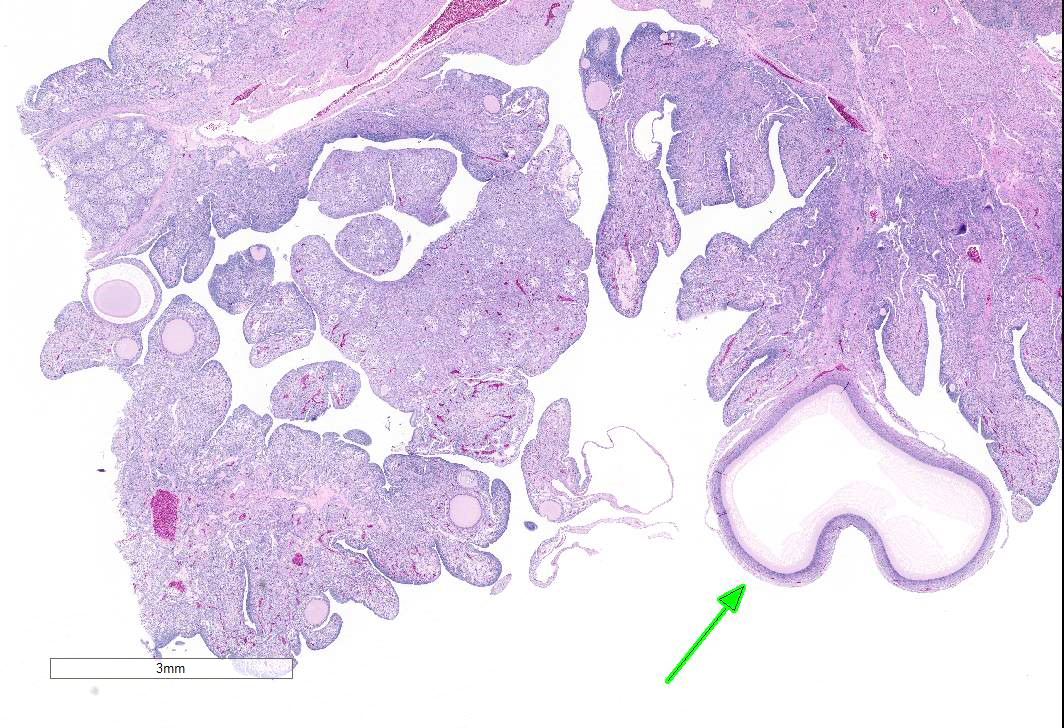
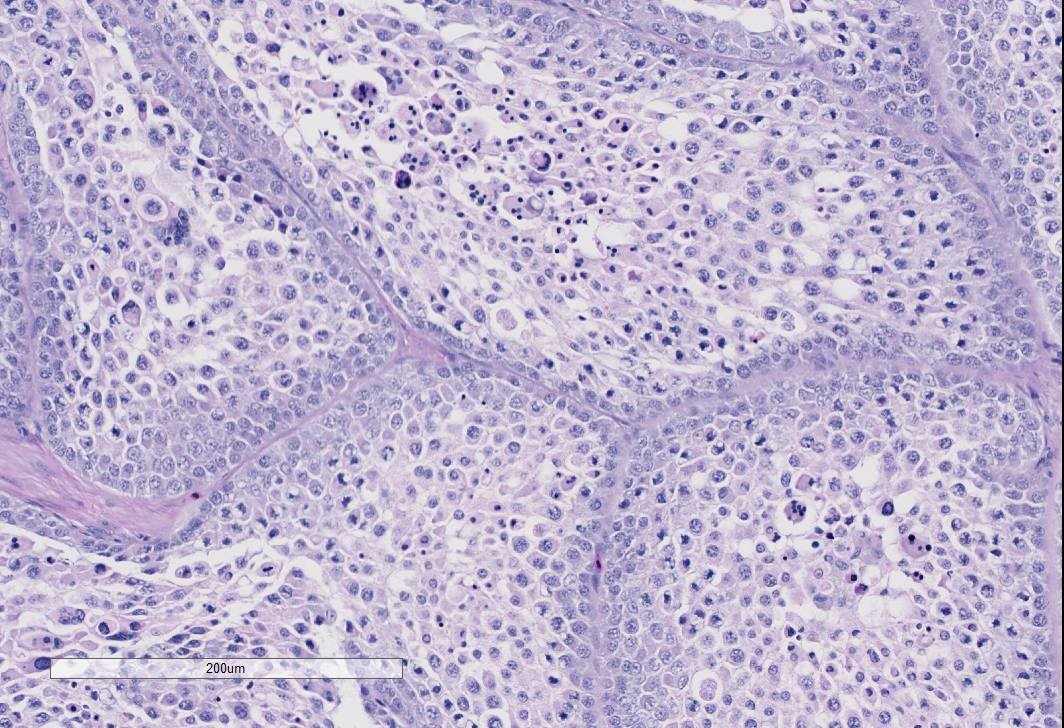
The examined gonad is comprised of both ovarian and testicular tissue, with variable degrees of differentiation. The ovarian tissue is characterized by fibrovascular stroma and finger-like projections (cortex) in which a low number of small developing follicles and one megalethical ovum are suspended. Throughout the ovarian stroma are observed multiple aggregates of lipid-laden cells (vacuolar cells), a few atretic follicles, and low numbers of scattered granulocytes and a few pigment-laden cells. Testicular tissue is either embedded in ovarian stroma with or without encapsulation, or attached to the ovarian tissue by a thin stalk of fibrous connective tissue. The testicular tissue is characterized by numerous, sometimes irregularly shaped tubular structures (seminiferous tubules) of varying sizes that are supported by fine to dense fibrovascular stroma. In the areas where the testicular tissue is well-demarcated/encapsulated, the tubules are fairly well-differentiated and contain developing spermatogonia, occasional multinucleated cells, and a few cells with pyknotic nuclei. Few to no late spermatids or spermatozoa are present. Adjacent to the encapsulated nodule of testicular tissue, there is a small collection of tubules lined by tall columnar epithelium (presumptive vestigial epididymis) which are devoid of spermatozoa.
Normal adrenal gland is also present along one side of the section.
Contributor’s Morphologic Diagnosis:
Gonad: Ovotestis
Contributor’s Comment Disorders of sexual development (DSDs) are relatively common and affect all domestic animal species.10 The gonads, reproductive tract or external genitalia may be affected. The term intersex has been traditionally used to describe animals which exhibit some of the characteristics of both sexes.7 The incidence of intersex in commercial poultry flocks has been estimated at 0.05%,1 and naturally occurring sex reversal has been reported in multiple avian species.1,4,9 Apparently, birds are more prone to the development of these condition for two reasons: firstly, the capability that both male and female embryos have, where cortical and medullary tissue in the developing gonads can later develop into ovary and testis respectively, is maintained after hatching.5 Secondly, there is a unique asymmetry during embryogenesis in female birds,2 in which greater numbers of primordial germinal cells migrate to the left gonad than to the right, that persist throughout life.4 Sex reversal most commonly occurs in mature female birds that have had a pathologic condition resulting in atrophy of the ovary with subsequent development of seminiferous tubules in the normally rudimentary right gonad or in the medulla of the atrophic left ovary,5 and this has been experimentally reproduced after gonadectomy.2 Mammals or birds with both ovarian and testicular tissue are classified as hermaphrodites (as opposed to pseudo-hermaphrodites which have the gonads of one sex and duct systems, external genitalia and some sexual characteristics of the opposite sex).8 The ovotestis presented here is the left gonad of an adult chicken presented to us as one of three mature Sussex hens. There was evidence of follicle development and a follicular hierarchy in the ovotestis, with the larger follicles having a stigma. However, the largest follicle was about one third of the size of those of commercial layers. The oviduct was developed to about 50% of the size of a normal fully functioning oviduct. Unfortunately, it is not known if this chicken had ever laid any eggs. The testicular tissue in our case was present in the ovarian medullary stroma, sometimes well circumscribed and encapsulated, but in one area was separated from the ovary, attached only by a thin stalk of fibrovascular tissue. Interestingly, a second chicken from this batch of three, also had an ovotestis. A high incidence (2.4%) of hermaphrodites was recorded in a flock of Cochin bantams and it was suggested that there may be a hereditary predisposition for the condition.5 It is not known if our two Sussex chickens were related.
In the last decade, there have been efforts to better classify DSDs by including the sex chromosome type, presence of SRY (in mammals; birds do not have SRY), gonadal type, tubular genitalia and external genital phenotype. Chromosomal typing was not performed in the present case but triploidy (ZZW) has been reported in some hens with ovotestis; triploidy can only develop at the time of fertilization.5,9
Mechanisms of sexual differentiation of the gonads appear to be highly conserved and many of the important signaling factors involved in ovarian or testicular development in mammals are also implicated in birds.2 While it is known that the embryonic gonads in birds are bipotential for a period of time, just as they are in mammals, the sex determining system has not been fully elucidated in birds.2 In mammals, the dominant acting gene is carried by the Y chromosome (SRY gene), and no matter how many X chromosomes are present, a single Y chromosome dictates testicular development and a genetic male gender.6 In birds, the female is the heterogametic sex (ZW) while the male is homogametic (ZZ).2,3 Three possible mechanisms of sex determination in birds have been proposed: A - The W sex chromosome carries a dominant female determinant, B - Dosage of one or more Z-linked genes, and C - Both mechanisms are at play.2 The current weight of evidence tends to more strongly support mechanism A. For example, there are no cases of spontaneous male to female sex reversal in birds, and the loss of estrogen production by the left ovary allows the right gonad to take on a testis-like appearance and produce testosterone.2
Experimental work has also suggested that endogenous estrogens can have a determinant effect and variations in the levels of the hormone in the embryo can produce different effects. Exposure to estrogens feminizes the reproductive tract of the male embryo, inhibition of estrogen in the female embryo disturbs development and can result in phenotypic sex reversal; both resulting in the formation of an ovotestis.2,3
Contributing Institution:
University of Connecticut
Connecticut Veterinary Medical Diagnostic Laboratory
Department of Pathobiology and Veterinary Science
College of Agriculture, Health and Natural Resources
JPC Diagnosis: Gonad: Ovotesis.
JPC Comment: The contributor has done an excellent job in reviewing the normal sexual development of the chicken as well as abnormal sexual development. In chickens and all other birds, a ZZ male/ZW female sex chromosome system exists. Until day six post-fertilization, male and female gonads are morphological indistinguishable; at this point, ZZ males develop bilateral testes and in ZW females, the left gonad develops into a functional ovary. In the developing ovary, expression of the P450 cytochrome enzyme aromatase (secreted within the ovarian medulla) has been identified as the major early determinant of sexual differentiation, as its secretion catalyses estrogen secretion and subsequent ovarian development. In the male, the enzyme SOX9, expressed within the same window, will trigger Sertoli cell differentiation and testicular development. Treatment of chicken embryos in early development with aromatase inhibitors will result in female to male sex reversal.7
References:
- Abdel-Hameed F, Shoffner RN. Intersexes and sex determination in chickens. Science 1971; 172:962-964.
- Ayers KL, Smith CA, Lambeth LS. The molecular genetics of avian sex determination and its manipulation. Genesis 2013; 51:325-336.
- Brunstrom B, Axelsson J, Mattsson A, Halldin K. Effect of estrogens on sex differentiation in Japanese quail and chicken. Gen Comp Endocrinol 2009; 163:97-103.
- Chalmers GA. Ovotestes and sexual reversal in racing pigeons. Can Vet J 1986; 27:82-84.
- Fitzgerald SD, Cardona CJ. True hermaphrodites in a flock of Cochin bantams. Avian Dis 1993; 37:912-916.
- Kumar V, Abbas AK, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2015.
- Lambeth LS, Cummins DM, Doran TJ, Sinclair AH, Smith CA. Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos. PLOS One 2013, 8:6:e68362.
- McGeady TA, Quinn PJ, Fitzpatrick ES, Ryan MT, Kilroy D, Lonergan P. Veterinary Embryology. Chichester, England: Wiley-Blackwell; 2017.
- Ohno S, Kittrell WA, Christian LC, Stenius C, Witt GA. An adult triploid chicken (Gallus domesticus) with a left ovotestis. Cytogenetics 1963; 2:42-49.
- Schlafer DH, Foster RA. Female Genital System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 3. Philadelphia, PA: Saunders Elsevier; 2007:358-464.