Signalment:
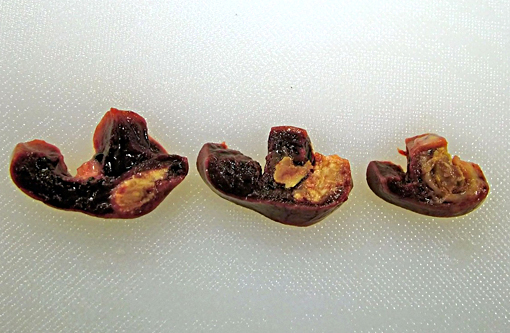
Gross Description:
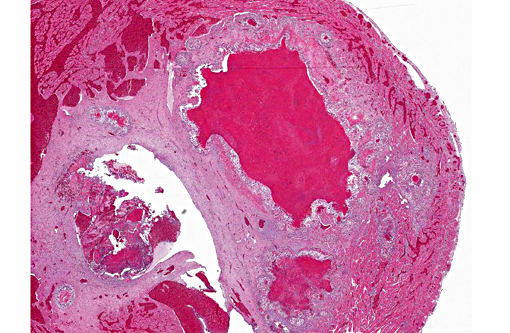
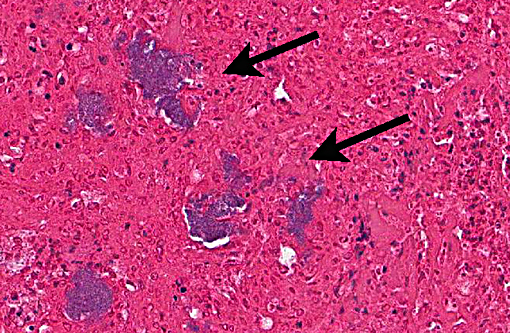
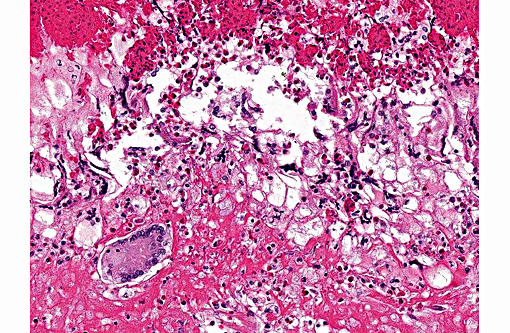
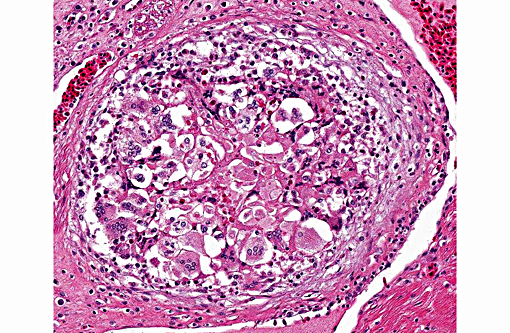
Histopathologic Description:
Morphologic Diagnosis:
1. Heart, right ventricle: Mural and valvular vegetative endocarditis with heterophilic and granulomatous inflammation and intralesional small gram-negative cocci; etiology, Neisseria iguana
2. Heart, right ventricle: Myocardial granulomas
Lab Results:
Condition:
Contributor Comment:
In the 1980s, a syndrome of abscesses and septicemia in rhinoceros iguana and common iguana was identified at the National Zoological Park.(7) The causative agent of the disease was classified as a new bacterium called Neisseria iguanae.(1) The bacterium was also isolated from the oral cavity from healthy animals in the collection. The cutaneous abscesses were believed to be the result of N. iguanae infection of the skin following bite wounds. One of the iguanas developed N. iguanae septicemia manifested as a liver abscess. In this case, the intraspecies aggression between the three common iguanas most likely resulted in the cutaneous abscess and mural and valvular endocarditis caused by Neisseria iguanae in this case.
The pathogenesis of bacterial endocarditis (also classified as infective endocarditis) is complex.(4,8,9,10) The formation of infective endocarditis lesions involves the preparation of the endothelial layer for colonization, adherence of bacteria to the endothelial surface and survival of the bacteria with propagation of the thrombus.(8) Intact endothelium is believed to be resistant to bacterial colonization.(4,8,9,10) The resistant endothelial layer has to be disturbed in order for bacteria to adhere. The disturbance of the endothelial layer can be the result of mechanical forces or due to endothelial cell activation and damage as the result of local proinflammatory molecules such as IL-1.(10) The endothelial cell damage causes activation of the coagulation cascade through the activity of tissue factor resulting in what is termed nonbacterial thrombotic endocarditis (NBTE). The resulting thrombus is colonized by bacteria that can adhere to damaged endothelial cells, platelets and adhesive extracellular matrix molecules such as fibrin and fibronectin. The bacteria adhere to the matrix molecules of the clot using a variety of surface molecules collectively called microbial surface component reacting with adhesive matrix molecules (MSCRAMMs).(10) The proliferation of the thrombus of infective endocarditis involves the interaction of bacterial pathogens and the host immune and coagulation systems. Infective endocarditis in the right side of the heart can result in emboli showering the lungs.(9) Infective endocarditis in the left side of the heart can result in systemic embolism.
JPC Diagnosis:
Conference Comment:
Conference participants described this lesion as a severe granulomatous endocarditis with granuloma formation and fibrosis. The ventricle was described as being 100% occluded by a large, dense fibrin thrombus which contains numerous bacterial colonies as well as erythrocytes and necrotic debris, and is multifocally attached to the markedly thickened endocardium. Multiple granulomas are present in the superficial myocardium, near the epicardialmyocardial junction. Granulomas contain a dense core of eosinophilic debris (characteristic of reptile granulomas) surrounded by multiple macrophages with the presence of many multinucleate giant cells as well. The abundant white space surrounding the dense central core of debris is likely the result of retraction artifact. The granulomas most peripheral layer is composed of dense fibrous connective tissue. The differential diagnosis discussed includes mycobacterial and fungal infections.
The moderator briefly discussed the structure of reptile hearts as there are significant differences with mammalian hearts. Most reptiles have a single common ventricle and two atria. Three cavities or divisions are present in the ventricle, termed the cavum pulmonale, cavum arteriosum and cavum venosum and are partially separated by muscular septa. Blood flows from the right atrium, through the cavum venosum and into the cavum pulmonale and then enters the pulmonary circulation. Oxygenated blood flows from the pulmonary veins and reenters the heart through left atrium, flows into the cavum arteriosum during diastole, which channels blood into the cavum venosum which then flows into the aorta. Oxygenated and deoxygenated blood is separated by pressure differences, outflow resistance and differential flow. Shunting and mixing of oxygenated and deoxygenated blood is variable depending on the reptile species and activity level. Nonetheless, blood flows are described as well separated within the ventricle (due to septa) and mixing of oxygen-poor and oxygen-rich blood is minimized.(4)
References:
1. Barrett SJ, Schlater LK, Montali RJ, Sneath PHA. A new species of Neisseria from iguanid lizards, Neisseria iguanae sp. nov. Lett Appl Microbiol. 1994; 18:200-202.
2. Freedman LR. The pathogenesis of infective endocarditis. J Antimicro Chemother. 1987; 20(Suppl. A): 1-6.
3. Hung MC, Christodoulides M. The biology of Neisseria adhesins. Biology. 2013; 2(3):1054-1109.
4. Jensen B, van den Berg G, van den berg R, Oostra RJ et al. Development of the hearts of lizards and snakes and perspectives to cardiac evolution. PLoS One. 2013; 8(6):e63651.
5. Liu G, Tang CM, Exley RM. Non-pathogenic Neisseria: members of an abundant, multihabitat, diverse genus. Microbiology. 2015; 161(7):1297-312.
6. Markey B, Leonard F, Archambault M, Cullinane A, Maguire D. Glucose nonfermenting, Gram-negative bacteria. In: Clinical Veterinary Microbiology. 2nd ed. London, UK: Mosby Elsevier; 2016: 375-379.
7. Plowman CA, Montali RJ, Phillips LG, Schlater LK, Lowenstine LG. Septicemia and chronic abscesses in iguanas (Cyclura cornuta and Iguana iguana) associated with Neisseria species. J Zoo Anim Med. 1987; 18(2-3): 86-93.
8. Sullman PM, Drake TA, Sande MA. Pathogenesis of endocarditis. Am J Med. 1985; 78 (Suppl. 6B): 110-115.
9. Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc Pathol. 2006; 15: 256-263.
10. Widmer E, Que Y-A, Entenza JM, Moreillon P. New concepts in the pathophysiology of infective endocarditis. Curr Infect Dis Rep. 2006; 8(4): 271-279.