CASE II: 73640 (JPC 4166754)
Signalment:
14-year-old female African Penguin, (Spheniscus demersus)
History:
This penguin, which was housed in an indoor-outdoor zoological exhibit, presented to veterinary staff for anorexia of 5-days duration. Initial treatments included gavage feeding with Piscivore liquid nutrition, subcutaneous fluids, metoclopramide, maropitant and itraconazole (for possible aspergillosis). Anorexia persisted over the next four days despite supportive care. Four days after presentation, slightly labored breathing was noted, and terbinafine and enrofloxacin were added to the medications. Over the following week, the penguin regurgitated all offered fish, but responded well to gavage feeding and maintained body weight. A radiograph taken on day eight revealed a radiodense foreign body in the caudal ventriculus. Multiple hematology examinations performed during this time showed a decline in hematocrit without overt anemia (PCV remained within reference range) and no signs of infectious or inflammatory disease. While anesthetized for foreign body removal, the bird went into cardiac arrest, and resuscitation efforts were unsuccessful.
Gross Pathology:
At necropsy, the ventriculus contained a bullet measuring 1.5cm in length. The ingesta surrounding the bullet was discolored in grey. The mucosal surface appeared intact with no areas of ulceration. The kidneys and other visceral organs appeared grossly unremarkable.
Laboratory results:
Liver lead level:
48.24 mg/kg
Kidney lead level: 289.5 mg/kg
Microscopic Description:
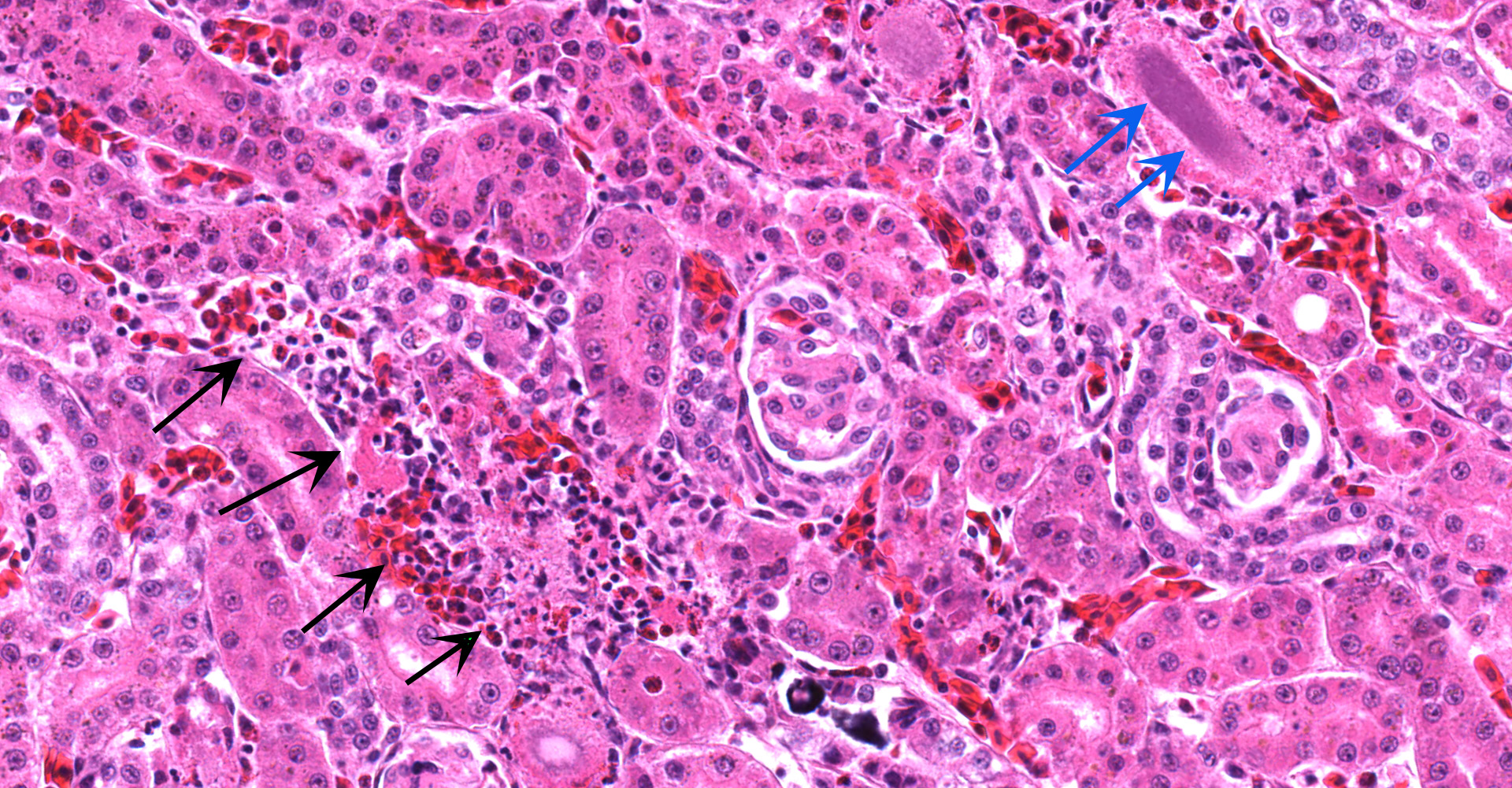
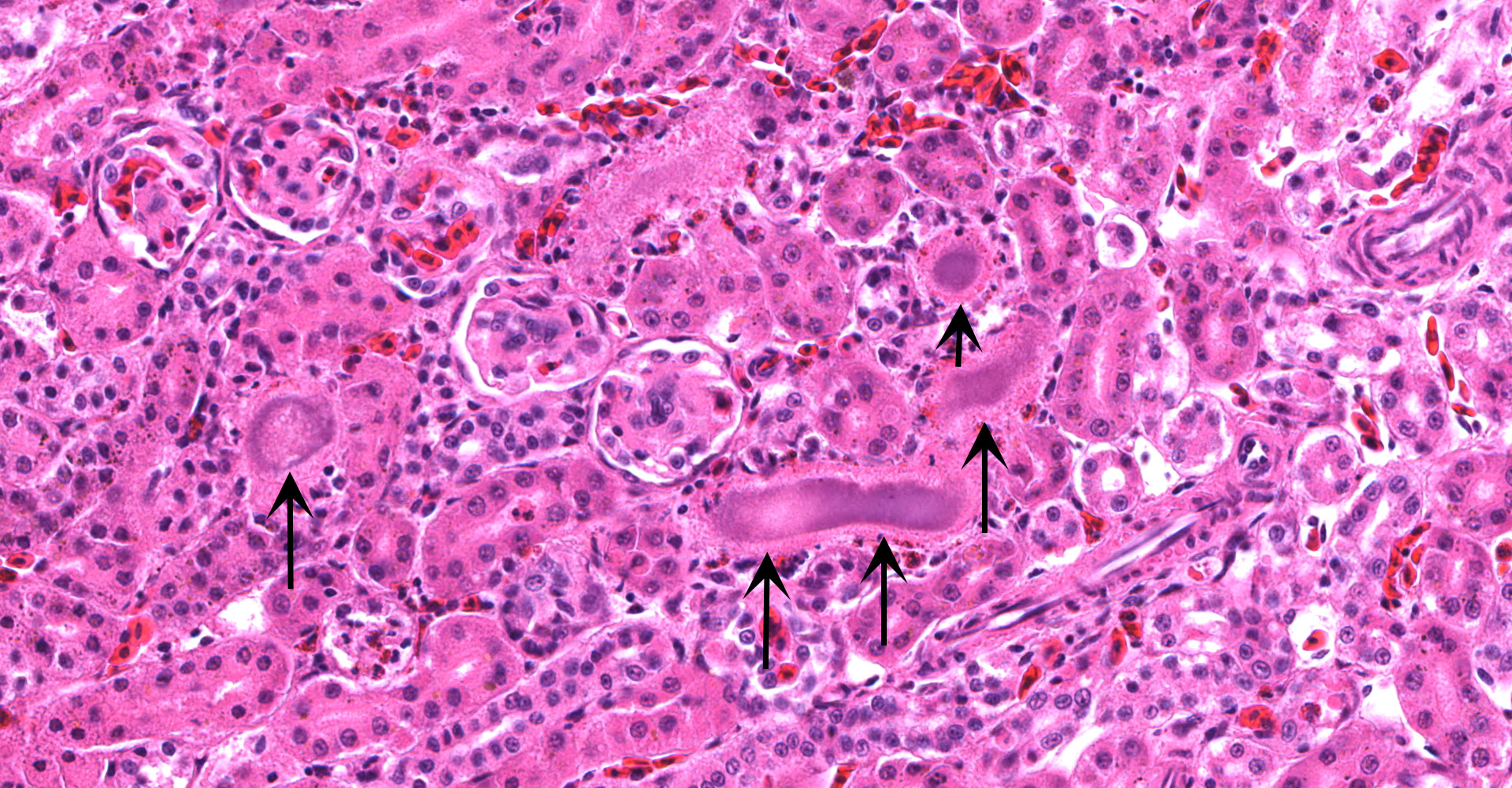
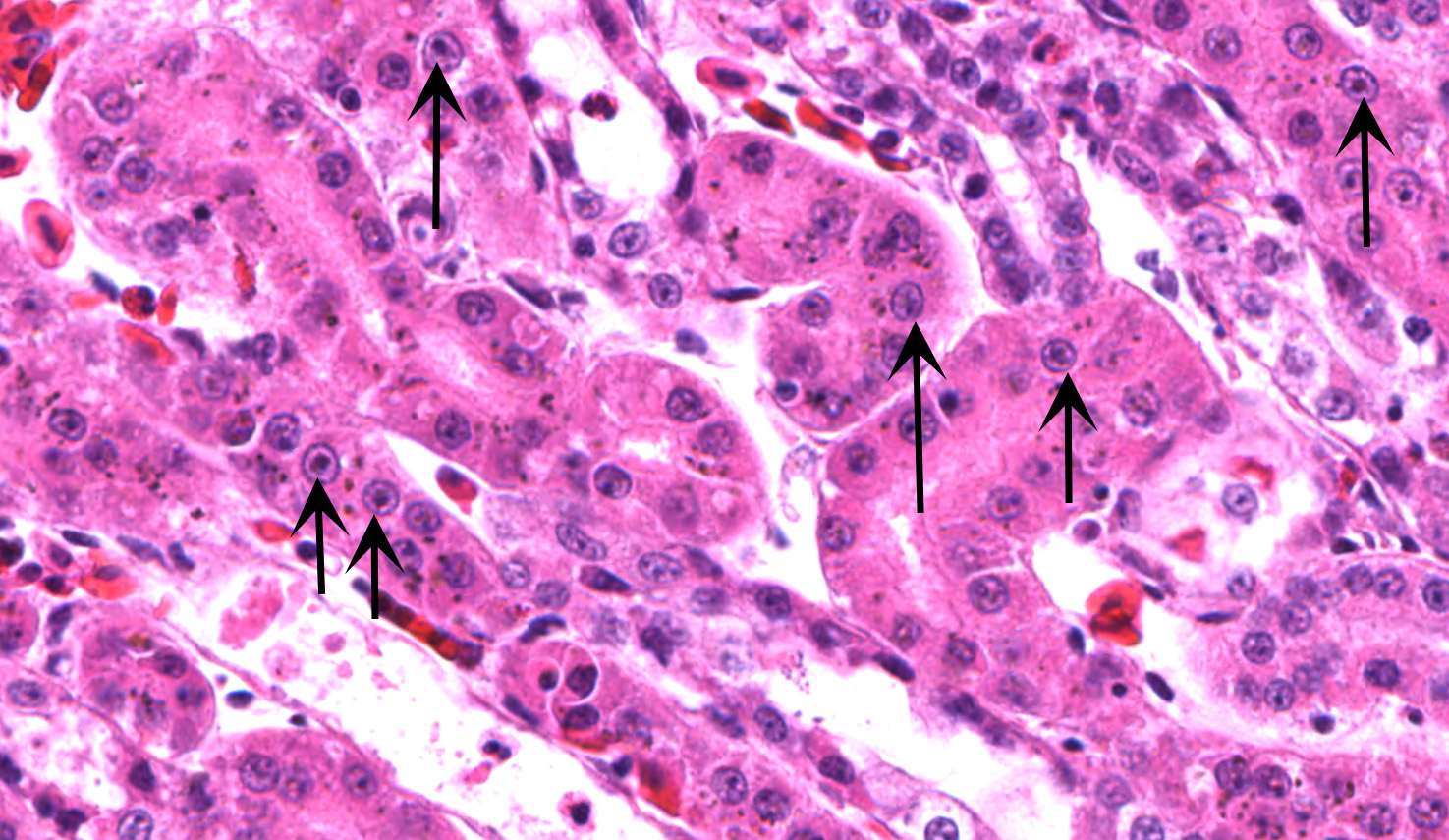
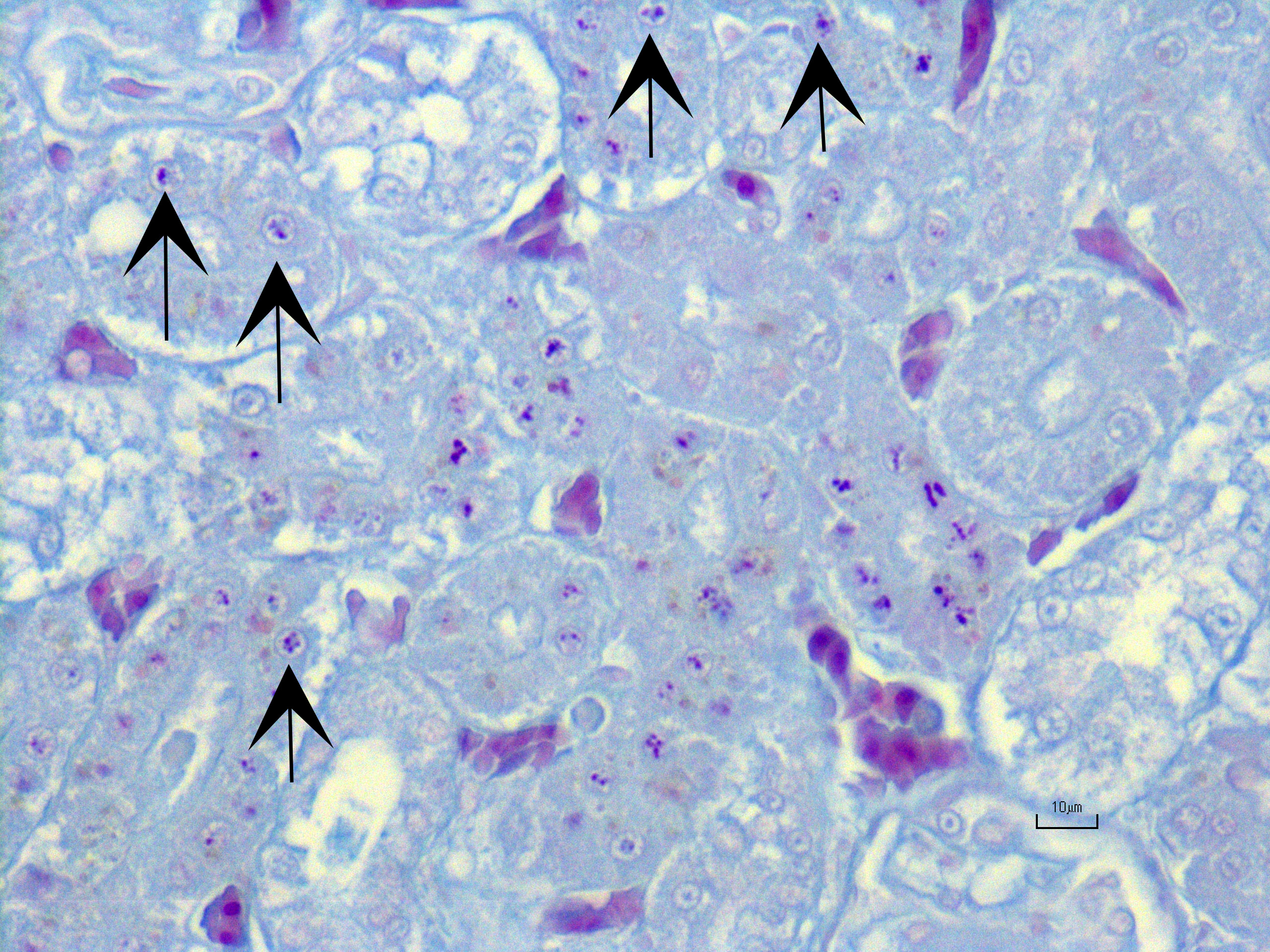
Kidney ? Multifocally, the proximal tubular epithelial cells are mildly swollen with vacuolated cytoplasm and vesicular nuclei (degeneration), or hypereosinophilic and discohesive with pyknotic or lytic nuclei (necrosis). Within the degenerate epithelial cells, nuclei frequently contain multiple, amphophilic, round inclusion bodies measuring approximately 1-3 µm in diameter. Necrotic tubules often contain aggregates of lightly-basophilic, finely granular to radiating, acicular material (consistent with early urate crystal deposition). The intranuclear inclusion bodies are strongly acid-fast positive.
Contributor?s Morphologic Diagnoses:
Kidney: Tubular degeneration and necrosis, multifocal, moderate with intranuclear acid- fast inclusion bodies, and intratubular urate crystal deposition.
Contributor?s Comment:
Lead toxicosis is a well-recognized cause of morbidity and mortality in wildlife, humans and domestic animals. It is the most common form of heavy metal toxicity in birds, and is now thought to be the most frequent poisoning caused by a contaminant in avian species worldwide.6 Common sources of lead exposure in birds include ammunition, fishing sinkers, coins, contaminated sediment, as well as ingestion of affected animals (i.e. secondary poisoning).
Lead is capable of causing lesions and dysfunction in cardiovascular, neurological, hematopoietic, gastrointestinal, reproductive and immunological organs. Although the mechanism of lead-induced toxicity is not completely understood, the main targets of lead are enzymes involved in heme synthesis as well as thiol-containing antioxidants and enzymes (superoxide dismutase, catalase glutathione peroxidase, glucose 6-phosphate dehydrogenase, glutathione).4 Low levels of lead in blood and tissue are sufficient to inhibit the activity of these enzymes, causing various effects, including impaired heme synthesis and generation of reactive oxygen species (ROS). Additionally, lead inhibits and mimics the action of calcium, thus disrupting calcium-dependent pathways.
Generally, kidney and liver concentrations of lead above 36mg/kg on a dry weight basis are considered diagnostic for lead toxicosis in animals (per Puls, R. (1994) Mineral Levels in Animal Health. Diagnostic Data. 2nd Edition, Sherpa International, Clearbrook, 82.). Liver and kidney lead concentrations in this African Penguin were 48.24mg/kg and 289.5mg/kg, respectively, confirming lead toxicosis. Lead tissue concentrations (on a fresh weight base) and their clinical relevance in birds have also been reported (Table 2). Necropsy lesions can be absent or include pectoral atrophy, fat depletion, esophageal/ proventricular impaction, ventricular erosions, gallbladder distension and lead objects in gastric contents.5 In the present case, the clinical presentation, pathology findings and toxicology results corroborate that this African penguin had a lead toxicosis. Due to the inhibition of heme synthesis, nonregenerative anemia is a common finding in lead toxicosis. Although this African penguin did not show profound anemia, the declining PCV was interpreted as a result of lead toxicosis. Notably, there were histologic changes in the kidney that are characteristic of lead toxicosis, including epithelial cell degeneration in the proximal convoluted tubules and intranuclear acid-fast inclusions.1
|
Lead Tissue Concentrations (Liver, Kidney) |
Clinical Significance |
|
<2mg/kg ww |
Background level |
|
2-6mg/kg ww |
Subclinical poisoning |
|
6-15mg/kg ww |
Clinical poisoning |
|
>15 mg/kg ww |
Severe clinical poisoning and death |
|
27 and 107 mg/kg ww |
Chronic poisoning |
Contributing Institution:
Johns Hopkins University, School of Medicine
Department of Molecular and Comparative Pathobiology
Broadway Research Building, #811
733 N. Broadway
Baltimore, MD 21205
Phone: 443-287-2953
Fax: 443-287-5628
JPC Diagnosis:
1. Kidney, tubules: Eosinophilic intra-nuclear inclusions.
2. Kidney, tubules: Nephritis, heterophilic, multifocal, mild, with gouty tophi and tubulorrhexis.
3. Liver, Kupffer cells and hepatocytes; kidney, tubular epithelium: Siderosis, diffuse, mild to moderate.
JPC Comment:
The recognition of plumbism (i.e. lead poisoning) has a long history, with the oldest known written description attributed to the Greek physician Nicander during the second century B.C.E. Multiple notable Romans also wrote about the dangers of lead, including Pliny, who described the dangers of red lead (lead oxide) and lead acetate . Ironically, Pliny also described how the lining copper pots with lead prevented the leaching of the copper into food. He also endorsed the reduction of grape syrup in lead vessels to produce sapa, a syrup used to sweeten wine and preserve fruit, a process that inherently resulted in the addition of lead acetate to the liquid.2 Debate continues as to the role of lead in the fall of the Roman Empire, which is often attributed to the lead used in Roman water pipes. However, the main source of lead for the Romans was probably sapa.2,7
The digestive tract is the primary site of lead?s entry into the body, with most lead absorption occurring in the small intestine.7 Inhaled fumes from heated lead and very fine particles (<0.5 µm) can enter via alveoli and be absorbed while larger particles are entrapped and cleared from the airway by mucociliary action. These particles are in turn swallowed and absorbed via the gastrointestinal tract. Risk factors for increased absorption of ingested lead include a high fat diet, mineral deficiency, and young age. A lipid rich diet and mineral deficiency (e.g. calcium) can increase lead absorption by 7- and 20-fold, respectively. There is also species variation, as monogastric animals absorb approximately 10% of ingested lead while less than 3% is absorbed in ruminants. However, up to 90% of ingested lead may be absorbed in young animals can absorbed. Once absorbed, the majority of lead is carried on erythrocyte cell membranes, with a lesser amount bound to protein or sulfhydryl compounds with only a scant amount found free in the serum. Lead is then distributed throughout the body, crosses the blood-brain-barrier, and also accumulates in bone. Lead deposited in bone typically has a low turnover rate. However, significant metabolic events, such as pregnancy and lactation, or chelating agents can result in rapid mobilization of deposited lead.7
As noted by the contributor, lead toxicity commonly affects avian species, including bald eagles. Plumbism in this species is primarily associated with the scavenging carcasses of animals killed with lead based ammunition and was found to be associated with 63.5% of diagnosed poisonings between 1975 and 2013 in a recent retrospective study.3 Of 93 specimens with blood levels consistent with severe lead poisoning (≥1 ppm), gross lesions were most frequently observed in the heart (51/93 cases) with multifocal myocardial pallor and rounding of the apex. Gross lesions were also observed in the brain (19/93) with petechiae or hemorrhagic necrosis. Histologic lesions were most commonly noted within the heart (76/93), followed by the brain (59/93), and the eyes (24/87). Histologically, the lesions were characterized by fibrinoid necrosis of small to medium caliber arteries, predominantly within the previously identified organs, with the gross and histologic lesions consistent with ischemia caused by a primary vascular insult. As a result, the authors concluded the presence of these lesions in the absence of infections agents or significant inflammation is suggestive of lead toxicity. As observed in this case, renal tubular degeneration and necrosis was also found to be rare in bald eagles, and was minimal to mild when present. Intranuclear lead inclusion bodies were not detected in the aforementioned study. Definitive diagnosis of lead toxicity is preferably done utilizing liver and/or kidney tissue with liver lead concentrations greater than 6ppm (wet weight) thought to be associated with clinical toxicity and greater than 10ppm with severe poisoning in bald eagles, although levels less than 6 ppm were also found to be associated with severe or fatal lead toxicity.3
In this case, conference participants noted the presence of abundant golden-brown, globular intracellular pigment within numerous cells in various organs, particularly Kupffer cells. One differential diagnosis for this pigment was hemozoin, an insoluable ferriprotoporphyrin created as the result of malarial (i.e. Plasmodium sp.) digestion of hemoglobin within the host. In addition, participants noted dense aggregates of periportal cells most consistent with erythroid precursors (i.e. extramedullary hematopoiesis), although lymphocytic infiltration could not be completely rule out. Post-conference correspondence indicated the contributor performed a Prussian blue stain, confirming the pigment contained iron. Additional stains included Grocott?s methenamine silver, acid fast, and Gram stains; no infectious organisms were identified. The JPC was unable to repeat this battery or any additional stains as the result of this case being a completely digital WSC submission to maintain compliance with the Convention on International Trade in Endangered Species (CITES). The African Penguin is a CITES protected species under Appendix II.
References:
1. Breshears MA and Confer AW. The Urinary System. In Pathologic Basis of Veterinary Disease. 6th Edition. Mosby Elsevier; 2017: 617-681.
2. Jonasson ME, Afshari R. Historical documentation of lead toxicity prior to the 20th century in English literature. Hum Exp Toxicol. 2018;37(8):775-788.
3. Manning LK, Wünschmann A, Armién AG, et al. Lead Intoxication in Free-Ranging Bald Eagles ( Haliaeetus leucocephalus). Vet Pathol. 2019;56(2):289-299.
4. Nemsadze K et al. Mechanisms of lead-induced poisoning. Georgian Med News. Jul-Aug 2009; (172-173):92-6
5. Samour J. Toxicology. In Avian Medicine. 2nd edition. Mosby Elsevier; 2008: 269-281.
6. Stidworthy MF. And Denk D. Sphenisciformes, Gaviiformes, Podicipediformes, Procellariiformes and Pelecaniformes. In Pathology of Wildlife and Zoo Animals. 1st Edition. Academic Press; 2018: 653-686.
7. Thompson, LJ. Lead. In: Gupta RC, ed. Veterinary Toxicology Basic and Clinical Principals. New York, NY: Elsevier; 2007: 438-441