WSC Conference 13, Case 2
Signalment:
4-year-old, intact male rhesus monkey (Macaca mulatta)
History:
The animal was experimentally infected with Simian-Human Immunodeficiency Virus (SHIV) and had a two-month history of nasal discharge and coughing and intermittent diarrhea for over a year.
Gross Pathology:
The animal is in lean body condition. Upon opening the carcass, scant adipose stores are observed. Upon opening the peritoneal cavity, the gallbladder and common bile ducts are moderately thickened. The liver is diffusely slightly pale and on cut surface the bile ducts are prominent throughout. Upon opening the pleural cavity, the lungs are mottled a pink, pale tan with a speckling of red and purple. Upon cutting through the nasal cavity, the nasal turbinates on the right side appear somewhat thickened or inflamed. Lesions are not observed in the brain, heart, kidneys, spleen, pancreas, the entire male reproductive tract, and the entire gastrointestinal tract.
Laboratory Results:
Nasal cavity swabs: Staphylococcus aureus (MRSA positive).
PCR on lungs: Positive for Pneumocystis carinii.
PCR on common bile duct: Negative for Enterocytozoon bieneusi; positive for Cryptosporidium parvum.
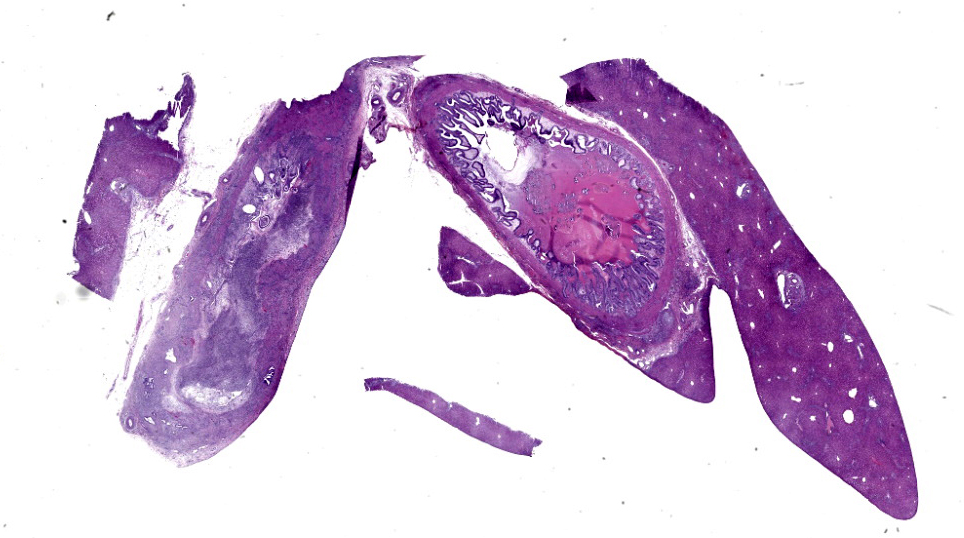
Microscopic Description:
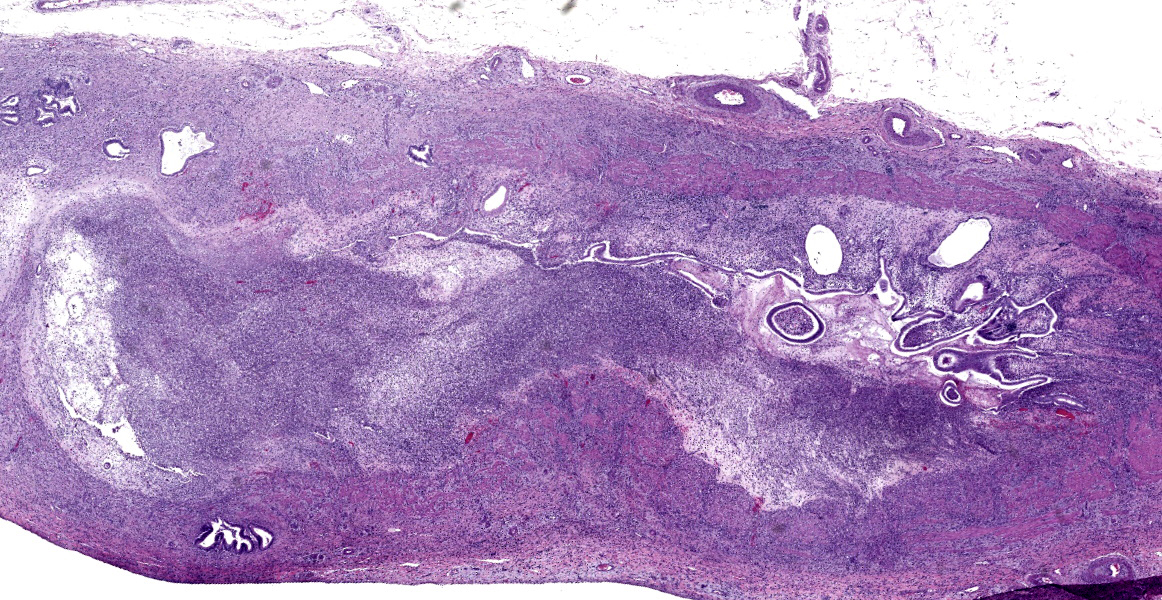
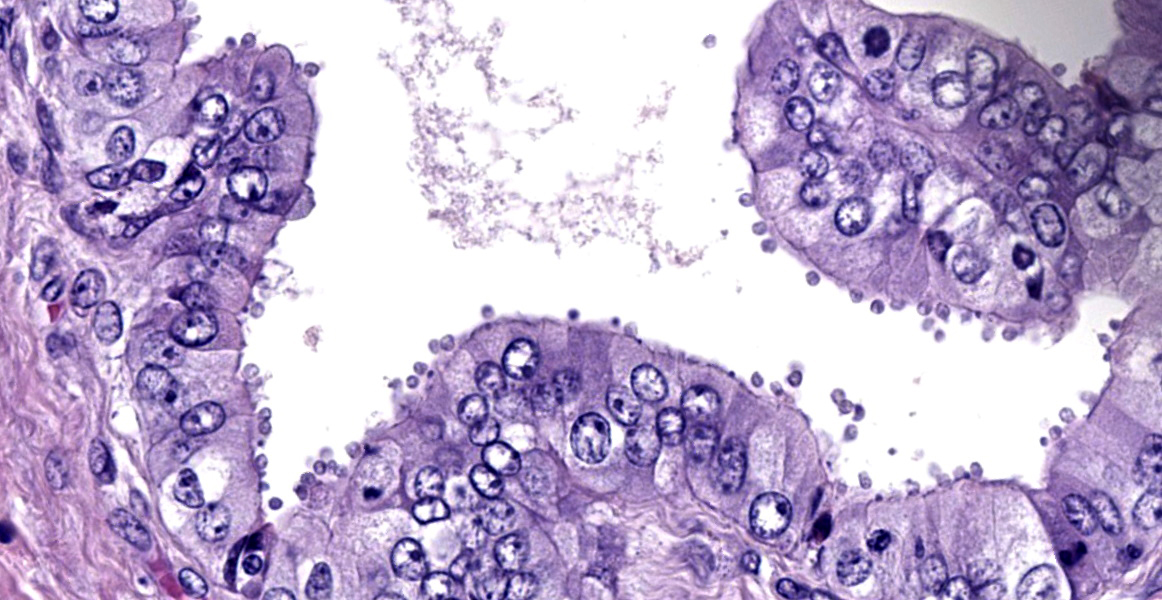
Common bile duct: There is a large area of mucosal and submucosal necrosis with abun
dant degenerate neutrophils and lesser macrophages. The remaining superficial mucosa is attenuated and the submucosa is edematous
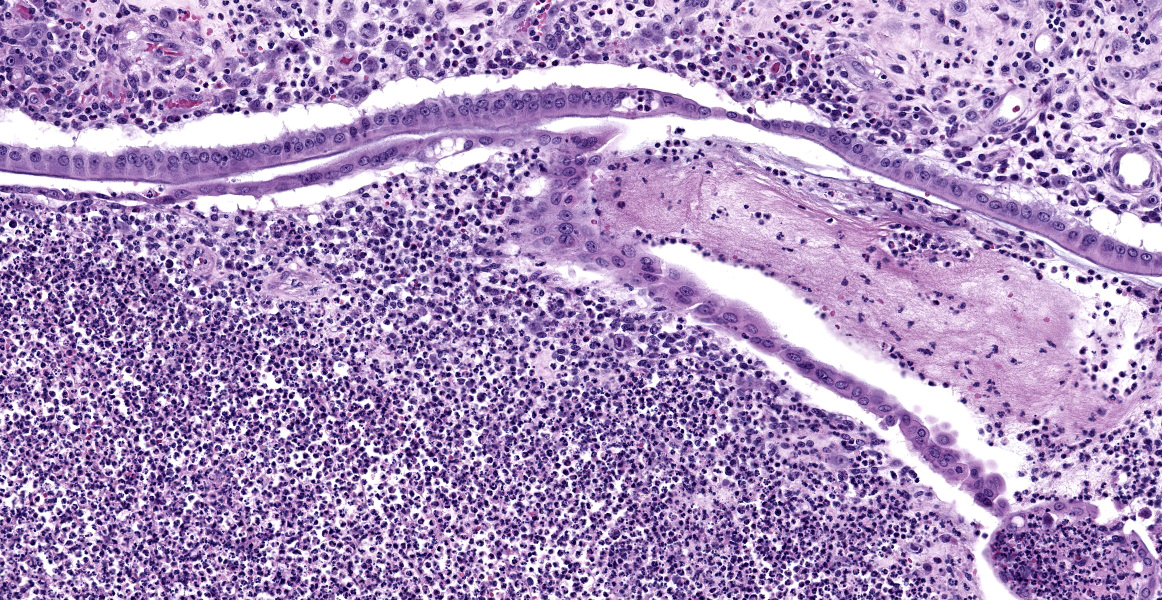
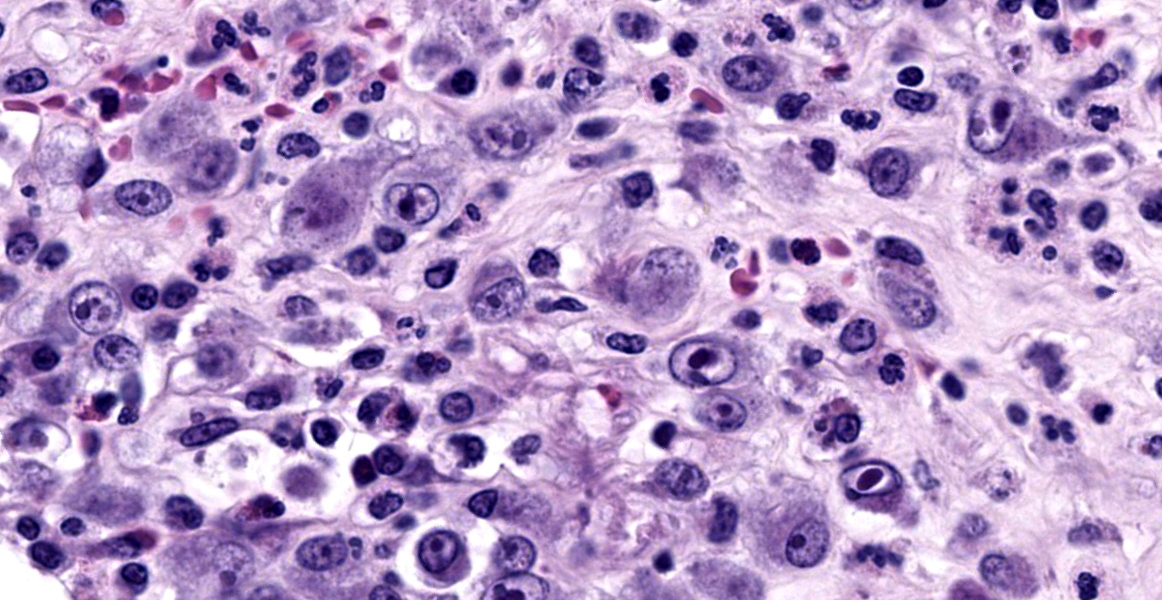
with abundant neutrophils, eosinophils, macrophages, and plasma cells. A few small areas of hemorrhage are observed. Areas of mucosal herniation through the muscle layers are observed. The smooth muscle has areas of atrophy and fibrosis with a mild eosinophilic and neutrophilic infiltrate. Numerous cells (smooth muscle, fibroblasts, epithelial cells) contain large basophilic intranuclear viral inclusion bodies. The mucosa has multifocal areas of 3-5 µm round protozoal organisms.
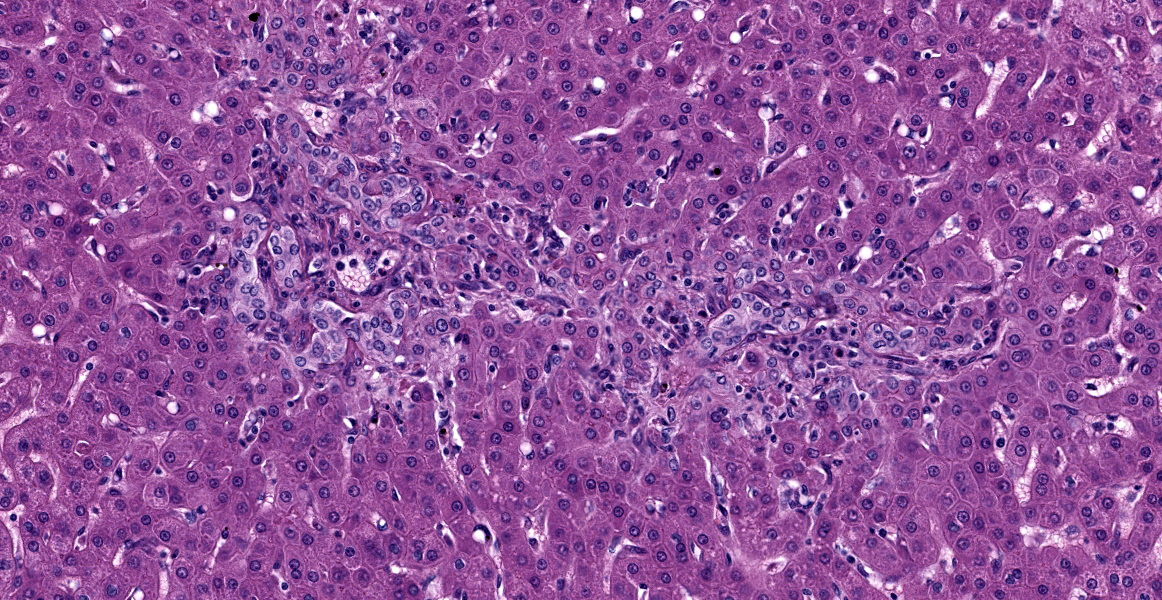
Liver: The portal areas have a mild interstitial fibrosis, mild to moderate biliary hyperplasia, a mild mixed inflammatory infiltrate, a few small areas of hepatocellular necrosis, and occasional biliary mucosal protozoal organisms.
Gallbladder: The lumen contains what appears to be a thick eosinophilic secretion admixed with mineralized and cellular debris. There are multifocal areas of submucosal edema. The mucosa has multifocal areas of protozoal organisms. The serosa has dense areas of plump fibroblastic proliferation admixed with numerous eosinophils.
Contributor’s Morphologic Diagnoses:
- Common bile duct: Cholangitis, necrotizing, severe, with intranuclear inclusion bodies consistent with cytomegalovirus and protozoal organisms consistent with Cryptosporidium.
- Liver: Hepatitis, portal, with biliary hyperplasia and Cryptosporidium.
- Gallbladder: Cholecystitis, with Cryptosporidium.
Contributor’s Comment:
This is a classic example of opportunistic infections in an immunocompromised rhesus macaque. The monkey was experimentally infected with Simian-Human Immunodeficiency Virus (SHIV). SHIV is a chimeric virus that contains HIV-1 viral envelope protein (Env) with a SIV backbone. The macaque adapted SIV (SIVmac) mirrors many of the key components of HIV-1 infection; however, there are differences between their Envs and the creation of a SIV virus with a HIV Env allows animal models to be used.2 SHIV leads to a severe decrease in CD4 lymphocyte populations allowing opportunistic bacteria, fungi, viruses, and protozoa to cause disease.
Cytomegalovirus (CMV) is a betaherpesvirus.1 CMV is ubiquitous in non-human primate populations. It has been identified in rhesus macaques (Macaca mulatta), Japanese macaques (Macaca fuscata), african green monkeys (Cercopithecine aethiops), drill monkeys (Mandrillus leucophaeus), squirrel monkeys (Saimiri sciureus), baboons (Papio sp.), marmosets (Saquinus fuscicollis, Callithrix jacchus), and chimpanzees (Pan troglodytes).1
In breeding colonies of rhesus macaques, 50% of infants are seropositive for CMV by 6 months of age and almost 100% are seropositive by 1 year of age. Horizontal transmission is believed to be from mother to infant through breast milk and saliva.1 Virus is also secreted in urine, semen, and cervical secretions. In immunocompetent animals, the virus has a predilection for salivary glands.1
In immunodeficient animals, as in human CMV, simian CMV can produce end-organ disease in the central and peripheral nervous system, lungs, lymph nodes, hepatobiliary system, gastrointestinal tract and arteries.3 CMV can be detected within multiple tissues or may be limited to a single site.1,3
The virus produces intranuclear (owl’s eye appearance) and intracytoplasmic inclusion bodies and induces tissue necrosis and neutrophilic infiltrate in immunosuppressed animals similar to that seen in human CMV. Cells most commonly infected include fibroblasts, epithelial cells, endothelial cells, smooth muscle cells, and macrophages.9
Cryptosporidium is a parasitic protozoa belonging to the phylum Apicomplexa.10 It is associated with enteric disease (diarrhea) in numerous species.5 Animals can be infected with multiple species of Cryptosporidium.10 Transmission is through the fecal-oral route and contact with animals, manure, or contaminated food and water.5 Transmission occurs through ingestion of oocysts which release sporozoites that infect intestinal epithelial cells. The parasite then replicates asexually in an intracellular but extracytoplasmic niche present at the luminal surface of intestinal epithelial cells. Lysis of the host cell releases merozoites that reinvade other epithelial cells. After three rounds of asexual replication, obligate sexual reproduction leads to generation of more oocysts which can hatch and reinfect within the same host or be shed into feces for transmission.4,5 In immunodeficient human hosts, infection leads to chronic disease, which can manifest as biliary involvement and sclerosing cholangitis. Cryptosporidium can also infect the respiratory tree, stomach, and pancreas.7,11 In human AIDS, cryptosporidiosis infection increases as CD4+ T-cell numbers decrease. Restoration of these cell numbers are associated with clearance of the parasite.4
Hepatobiliary dysfunction is common in HIV/AIDS. AIDS cholangiopathy (AC) is a well-documented biliary syndrome in severely immunocompromised AIDS patients. AC is a syndrome of biliary obstruction and
liver damage due to infection-related strictures of the biliary tract. AC is highly associated with opportunistic infections. These infections, in decreasing prevalence, are Cryptosporidium, cytomegalovirus, and Enterocytozoon bieneusi.6
Contributing Institution:
National Institutes of Health
Division of Veterinary Resources
Bethesda, MD
JPC Diagnoses:
- Common bile duct: Choledochitis, necrotizing, circumferential, chronic, diffuse, marked, with intranuclear karyomegalic viral inclusions and apicomplexan schizonts.
- Liver, bile ducts: Cholangiohepatitis, lymphoplasmacytic and eosinophilic, chronic, multifocal, mild, with biliary hyperplasia and apicomplexan schizonts.
- Gallbladder: Cholecystitis, lymphocytic and eosinophilic, diffuse, mild, with apicomplexan schizonts.
JPC Comment:
As the contributor notes, the pathology in this case is due to opportunistic pathogens emboldened by immunosuppression secondary to experimental SHIV infection. SHIV is a chimeric retrovirus consisting of an HIV envelope protein produced within an SIV carrier that is used for research into HIV-1 antibody-based vaccines, neutralizing antibodies, and other envelope protein-targeting strategies.
Retroviruses such as SHIV, SIV, and HIV take their family name from the presence of a reverse transcriptase within the virion that is encoded in the viral genome.7 Reverse transcriptase is an RNA-dependent DNA polymerase that transcribes in a “reverse” fashion from RNA to DNA. All replication-competent members of the retrovirus family contain a minimum armamentarium of three major genes: gag, pol, and env. Reverse transcriptase is encoded by pol (polymerase), which also encodes the key enzyme integrase.7 The gag (group-specific antigen) gene encodes structural proteins and the env (envelope) gene encodes an envelope transmembrane glycoprotein that is broken down into a surface protein subunit (SU) and a transmembrane subunit (TM), is displayed on the virion surface, and is responsible for entry into host cells.7 A fourth essential gene, pro, encodes a protease that cleaves viral precursor proteins and is expressed differently in various retroviruses.7
A mature retroviral virion includes a nucleocapsid containing the RNA genome and the enzymes reverse transcriptase, protease, and integrase, all of which are surrounded by an envelope studded with many SU and TM proteins. Upon encountering a host cell, the SU envelope glycoprotein makes first contact and initiates conformational changes within the TM subunit that leads to fusion of the viral and host cellular membranes and the extrusion of the nucleocapsid into the host cytoplasm. Once in the cytoplasm, reverse transcriptase co-opts the host cell replicative machinery and produces double-stranded DNA copies of the viral genome which are then integrated into random sites within the host’s chromosomal DNA by the viral enzyme integrase.7 Release of virions from the host cell typically occurs via budding from the plasma membrane.
Several retroviruses can induce oncogenesis via several mechanisms. Malignant transformation can occur when proviral DNA randomly inserts near host genes that regulate the cell cycle (proto-oncogenes). The promoter and enhancer DNA that is part of the viral insertion may result in dysregulation of the cell cycle and promote tumorigenesis.7 It should be noted that this is a relatively rare occurrence as most viral genome integrations are innocuous. A second method of oncogenic transformation occurs when retroviruses contain viral oncogenes. It is thought that viruses acquire these viral oncogenes from host genomes during viral recombination events; typical viral oncogenes include tyrosine kinases, growth factors, growth factor receptors, transcription factors (v-myc) and G proteins (v-ras).7 These oncogenes are integrated into the host genome and exert strong effects on endogenous mitogenic signalling pathways, often resulting in oncogenesis. Bovine leukemia virus exemplifies a third method of oncogenesis that relies on a viral oncogene, tax, which upregulates both viral and host promoter sequences, no matter where on the genome tax is integrated (a mechanism termed trans-activation).7
SIV is one of many retroviruses of veterinary importance, many of which are well-studied and well-characterized. Examples include avian leukosis virus and retriculoendotheliosis virus, the oncogenic retroviruses of poultry; Jaagsiekte sheep retrovirus, enzootic nasal tumor virus, maedi-visna virus, and caprine arthritis-encephalitis virus of small ruminants; feline leukemia virus and feline immunodeficiency virus; bovine leukemia virus and bovine immunodeficiency virus; and equine infectious anemia virus.7
Conference discussion reviewed the SHIV animal model of HIV infection and the pathogenesis of HIV infection generally. The moderator admonished residents to remember that, while HIV famously infects and destroys CD4+ T lymphocytes, it also affects members of the monocyte-macrophage system, including microglia. Once infected, these cells secrete cytokines that ramp up the immune system, resulting in giant cell encephalitis, giant cell interstitial pneumonia, and granulmomatous lymphadenitis.
Dr. Alves drew participants’ attention to the expansion of the tunica intima that was a repeatable finding throughout the vasculature in section. Arteriopathy has long been associated with SIV and HIV infection, and there have been reports of cytomegalovirus infections that manifest with intimal thickening and fibrosis with varying degrees of vasculitis. Dr. Alves could not say with certainty which, if either, of CMV or SHIV was the source of the vascular changes, but reminded conference participants not to neglect the vasculature when evaluating lesions.
References:
- Barry PA, Chang WLW. Primate betaherpesviruses. In:Arvin A, Campadelli-Fiume G, Mocarski E, et al., eds. Human Herpeviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; 2007:1-71.
- Bauer AM, Bar KJ. Advances in simian-human immunodeficiency viruses for non-human primate studies of HIV prevention and cure. Curr Opin HIV AIDS. 2020;15(5):275-281.
- Berg MR, Owston MA, Gauduin MC, et al. Cytomegalovirus hypophysitis in a simian immunodeficiency virus infected rhesus macaque (Macaca mulatta). J Med Primatol. 2017;46(6):364-367.
- Cohn IS, Henrickson SE, Striepen B, Hunter CA. Immunity to Cryptosporidium; lessons from acquired and primary immunodeficiencies. J Immunol. 2022; 209:2261-2268.
- Khan SM, Witola WH. Past, current, and potential treatments for cryptosporidiosis in humans and farm animals: A comprehensive review. Front Cell Infect Microbiol. 2023; 13:1115522.
- Naseer M, Dailey F, Al Juboori, et al. Epidemiology, determinants, and management of AIDS cholangiopathy: a review. World J Gastroenterol. 2018;24(7):767-774.
- Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning S, Hartigan PJ. Veterinary Microbiology and Microbial Disease. 2nd ed. Blackwell Publishing, Ltd.; 2011:618-634.
- Reina FTR, Ribeiro CA, de Araujo RS, et al. Intestinal and pulmonary infection by Cryptosporidium parvum in two patients with HIV/AIDS. Rev. Inst Med Trop Sao Paulo. 2016;58:21-24.
- Sinzger C, Grefte A, Platcher B, et al. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major target organs of human cytomegalovirus infection in lung and gastrointestinal tissue. J Gen Virol. 1995; 76(4):741-750.
- Widmer G, Koster PC, Carmena D. Cryptosporidium hominis infections in non-human species: revisiting the concept of host specificity. Int J Parasitol. 2020; 50:253-262.
- Yanai T, Chalifoux LV, Mansfield KG, et al. Pulmonary cryptosporidiosis in Simian Immunodeficiency Virus-infected rhesus macacques. Vet Pathol. 2000;37 (5):472-475.