CASE IV: N9757432 (JPC 4101492)
Signalment:
7 year-old, male neutered, Labrador Retriever dog (Canis familiaris)
History:
A 7 year old Labrador Retriever presented to the Emergency Service for cluster seizures. He had a previous history of chronic, intermittent chewing behavior which had not responded to medical management with anticonvulsants, steroids, and pain relievers. On presentation, the patient was experiencing a generalized seizure which was mitigated with diazepam administration, however, focal facial seizures continued to occur. Following diazepam administration, the dog was laterally recumbent, poorly responsive, dehydrated and hyperthermic. He was admitted to the hospital for supportive care, anticonvulsant therapy, and a diagnostic workup. Overnight, he was treated with fluid therapy and long-acting anticonvulsants prior to being transferred to the Neurology service.
An MRI showed diffuse cerebral edema with herniation, and a right-sided mass effect, however, a discrete mass was not visualized with or without contrast enhancement. The dog was discharged, but remained dull with intermittent jaw chomping and required ambulatory support. The jaw chomping increased in frequency over the 6 weeks, after which the owners elected euthanasia.
Gross Pathology:
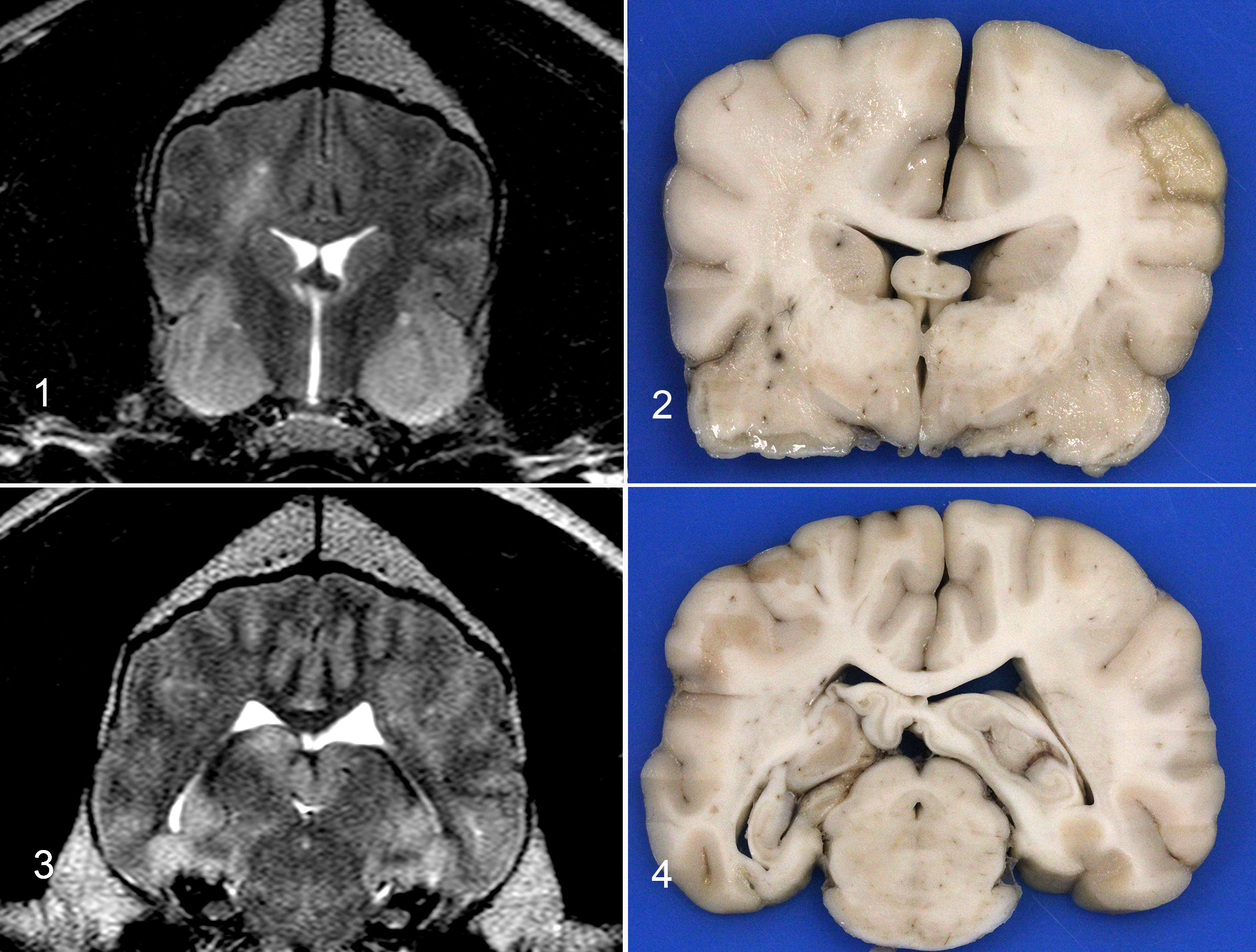
The brain is removed. The right side is more congested as compared to the left. No obvious masses are found with external evaluation. The occipital lobes are bilaterally mildly indented (presumed subtentorial herniation). Despite the lack of gross evidence of foramen magnum herniation, there is a locally extensive region of leptomeningeal hemorrhage at the brainstem, adjacent to the cerebellar vermis that measures 2.5 x 0.5 cm.
The fixed brain is sectioned. There is right and left sided expansion of the white matter. On the right side, there is cavitation of the centrum semiovale at the level of the caudate nucleus with hemorrhage and necrosis in the corresponding and contralateral pyriform lobes. On the left side, at the level of the thalamus and midbrain, there is white matter expansion with a mild midline shift and mottling of the cerebral cortex on the right and left sides. These images can be compared with the MRI imaging which shows hyperintensity of the right centrum semiovale and pyriform lobes and enlargement of the hippocampus with cerebrocortical hyperintensities.
Laboratory results:
History of elevated RMSF titers, antigen test negative.
CSF (6 weeks before necropsy): Neutrophilic pleocytosis
Microscopic Description:
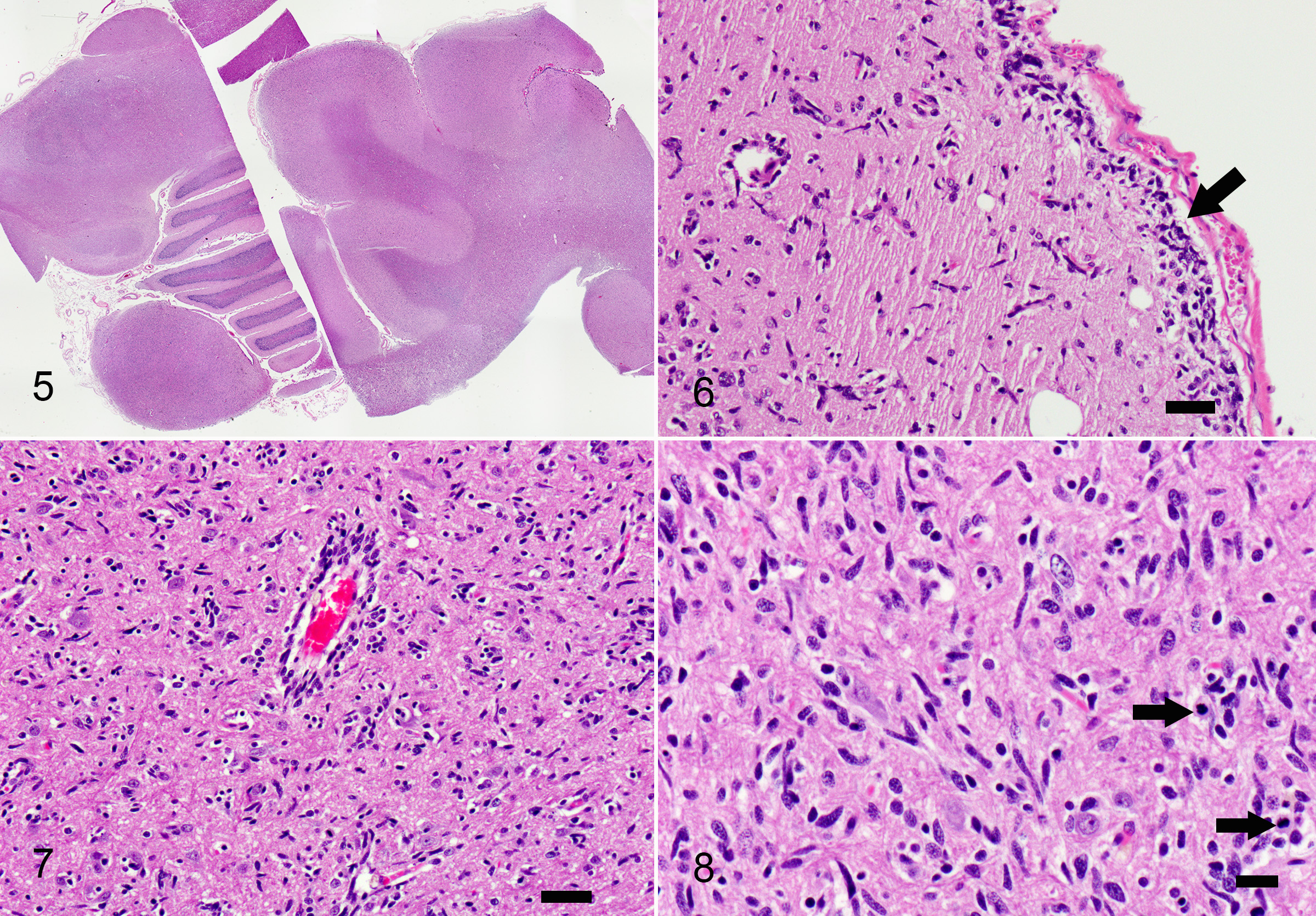
Samples include either one slide containing cerebral cortex and corpus callosum with medulla, cerebellum and caudal colliculus, or cerebral cortex, corpus callosum, hippocampus, thalamus and pineal gland.
Multifocally throughout the samples, in grey and white matter, there are populations of poorly defined, pleomorphic, spindle shaped cells, haphazardly infiltrating the neuroparenchyma with rare formation of parallel fascicles. These cells are markedly pleomorphic, with scant to moderate amounts of elongate, eosinophilic cytoplasm, and ovoid to slender or twisted nuclei. Nuclei exhibit up to 4-fold anisokaryosis, with coarse chromatin, frequently indistinct nucleoli, and an occasional large, magenta nucleolus. There are 10 mitoses in 10 high power fields, with variation throughout the samples. The spindle cells multifocally form aggregates in subpial and perivascular regions, with less frequent neuronal satellitosis. In the medulla, the nuclei are more intensely infiltrated by these spindle cell populations. In regions of higher cellularity and edema, there is rarefaction and malacia with gliosis including numerous gemistocytic astrocytes, Gitter cells, myelin vacuolation and axonal swelling (spheroid formation). Neuronal degeneration and loss are observed, most prominently in the hippocampus, with regional gliosis and gemistocytic astrocytosis.
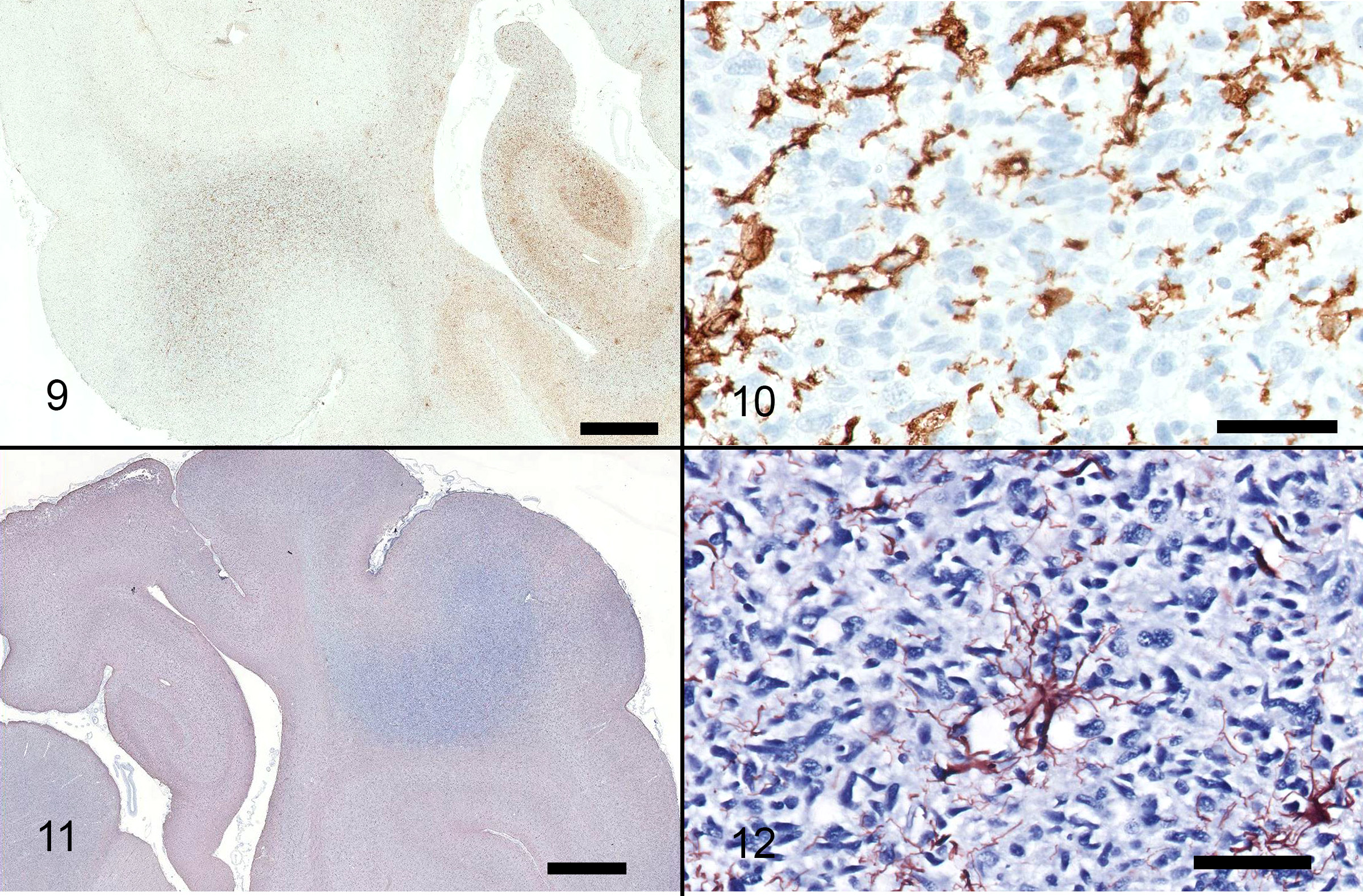
Immunohistochemistry for CD18, GFAP: CD18 staining reveals detectable antigen within cells in between the pleomorphic, spindle shaped cells, but does not stain the atypical cell population. GFAP reveals detectable antigen within astrocytes between pleomorphic spindle cell populations.
Contributor's Morphologic Diagnoses:
Brain (cerebrum, thalamus, midbrain, cerebellum): Gliomatosis cerebri (type I-like) with white matter cavitation, rarefaction, necrosis, gliosis with gemistocytic astrocytosis, spheroid formation, neuronal degeneration and loss.
Contributor's Comment:
The lesion in this case was characterized by widespread infiltration by a population of elongate, hyperchromatic cells, with no distinct mass formation. Multiple portions of the brain were affected, including multiple lobes within the cerebrum, thalamus, midbrain, cerebellum and medulla. These cells were present both on the right and left sides of the brain. The differential diagnoses included diffuse astrocytoma, microgliomatosis and more remotely, lymphoma, primitive neuroectodermal tumors (PNETs) and gliosis.3 Due to the widespread nature of the infiltration and presence of atypia within this population, gliomatosis cerebri (GC) was given primary consideration. Immunohistochemistry for GFAP and CD18 was negative in the atypical cell populations, which is supportive of GC. The infiltration was accompanied by marked white matter cavitation with malacia, rarefaction, neuronal loss and gliosis including gemistocytic astrocytosis. These findings are interpreted to be secondary.
Gliomatosis cerebri (GC) is a rare neoplasm of unknown cell origin but is classified as a glial cell tumor in the WHO classification of tumors of the nervous system.5,6,7 GC is a well-recognized entity in human medicine, characterized by widespread infiltration with preservation of the architecture of the brain.5,7 Gliomatosis in humans is divided into two subtypes. Type I is the most common and presents as a diffuse infiltration of the brain with no mass lesion. With type II gliomatosis, the diffuse infiltrate is accompanied by a mass lesion.1,6,7 Gliomatosis cerebri in dogs more commonly resembles type I GC,7 although multiple cases of type II-like GC in dogs were recently reported, and the authors recommended that canine GC should also be subclassified in this manner.1
The cell of origin is controversial. In humans, these neoplastic cells are immunoreactive for GFAP, which supports astrocytic differentiation,6 however, canine cases consistently do not exhibit immunoreactivity for GFAP, suggesting that these cells are not astrocytes.6 In one veterinary study, CD18 expression was observed in a subpopulation of neoplastic cells for one case, suggesting divergence along the monocyte/macrophage/microglial cell line, and a more accurate diagnosis of microgliomatosis.6 In another study, cases of canine GC were immunoreactive for Olig-2 and negative for GFAP, thus the authors suggested that GC may be a neoplasm of oligodendrocytes.1 Unfortunately, Olig-2 was not performed in this case.
Dogs with GC have been reported to range from 3 to 11 years of age, with clinical signs including general mentation changes, postural reaction deficits and cranial nerve dysfunction.6 The duration of these clinical signs is variable, ranging from 1 week to 6 months.7 In humans, common clinical symptoms include corticospinal tract deficits, dementia, headaches, seizures, mental and behavioral changes.7 In one study, four of six dogs with GC were males. No sex predilection is reported in people with GC.6,7 Bearded collies may be genetically predisposed to GC, as a cluster of cases have been observed in young siblings of multiple families.2
In reported cases of GC in dogs, there is variation in the area of brain involvement, with the cerebrum most consistently affected, and frequent concurrent brainstem involvement.7 In one case series, limited disease was reported in two cases; one with caudal brainstem and spinal cord involvement, and the other with spinal cord involvement at the level of T13 and L2.1,6 In humans, neoplastic infiltration typically involves two or more cerebral lobes, the corpus callosum, diencephalon, basal nuclei with variable brainstem involvement.6 In this case, multiple cerebral lobes were involved, as well as the corpus callosum, diencephalon and basal nuclei. In both humans and dogs, the white matter is typically more affected than the grey matter.7 A tropism for brain nuclei has been reported in canine cases, but is not recognized in humans.7 Additional common features include neuronal satellitosis, subpial and subependymal accumulations, perivascular cuffing, and a parallel arrangement of the neoplastic cells within white matter tracts. Subpial and perivascular aggregates were observed in this case.
Imaging findings with GC are nonspecific, making antemortem diagnosis and differentiation from other disease processes difficult.6 Histopathology is required for definitive diagnosis.6 In one study of seven dogs with GC, the neuroanatomic location, MRI and gross necropsy findings were suggestive of a focal lesion, however, diffuse CNS infiltration was documented with histologic examination.1 Minimal findings were observed on the MRI in many of the cases.1 Interestingly, an MRI performed on this patient 4 months earlier at another institution showed no appreciable abnormalities. An additional characteristic of GC is lack of contrast enhancement with MRI,1 also observed in this case. In the aforementioned study, neoplastic cells were present bilaterally even in cases with unilateral or no lesions identified with imaging. MRI was unable to predict lesion location in 6 cases, and all 7 cases had histologic lesions in regions that appeared grossly normal.1
Contributing Institution:
Animal Medical Center, 510 East 62nd St. New York, NY 10065
JPC
Diagnosis:
Cerebrum and brainstem: Astrocytoma, high grade, with diffuse gliomatosis cerebri-like infiltration.
JPC Comment:
This 2017 WSC submission includes an excellent description and overview of gliomatosis cerebri, a rare neoplasm of both canines and humans thought to be of glial cell origin.
The term "gliomatosis cerebri" was first used in human neuropathology in 1938 to describe CNS neoplasms with widespread infiltration of the telencephalic hemispheres. Over the following decades, the WHO Classification of Tumors of the Central Nervous System defined gliomatosis cerebri as a diffusely infiltrative neuroepithelial neoplasm with widespread involvement of more than two cerebral lobes with or without involvement of the deep gray matter structures (such as basal nuclei and thalamus), brainstem, cerebellum, and spinal cord. As discussed in greater detail below, the veterinary community is transitioning away from this grading scheme. Using this scheme, however, most gliomatosis cerebri cases are classified as a grade 2 or 3 glioma, occurring as either a primary or secondary gliomatosis cerebri. Primary gliomatosis cerebri are further classified as type I or II, as previously described by the contributor. Secondary gliomatosis cerebri are characterized by diffuse spread of neoplastic cells arising from a primary glioma. Despite aggressive treatment, including surgery, radiation, and chemotherapy, these neoplasms have a poor prognosis due to their clinical, morphological, and molecular heterogeneity which hinders attempts to assess the efficacy of specific therapies in humans. The vast majority of dogs are euthanized soon after diagnosis.8
Following this case's submission, additional tissues were separately evaluated as part of a case series evaluating the morphologic and immunohistologic characteristics of 24 cases of canine gliomatosis cerebri.8 Neoplastic cells from this case were immunoreactive to both GFAP and Olig2, confirming the contributor's suspicion of astrocytic origin based on cellular morphology.
Although a useful diagnostic aid, Olig2 and GFAP immunohistochemical stains are not specific for oligodendrocytes and astrocytes, respectively. However, strong diffuse nuclear immunoreactivity for Olig2 is highly consistent with glial histiogenesis. In the recent case series of 24 gliomas of diffuse growth pattern, neoplasms with oligodendrogial morphology had stronger and more widespread nuclear immunoreactivity to Olig2 associated with a less consistent or absent immunoreactivity for GFAP. In contrast, strong cytoplasmic GFAP immunoreactivity was evident and more consistent in neoplasms with astrocytic morphology as well with undefined gliomas. CNPase can be used to confirm oligodendroglial differentiation in cases in which morphology is consistent with glioma but Olig2 and GFAP are inconclusive.8
As alluded to above, the veterinary profession is transitioning away from "gliomatosis cerebri" as a distinct neoplastic entity. This shift is linked to the term's elimination from the 2016 WHO CNS tumor classification system due to lack of consensus in terms of definition, morphology, molecular profile, and treatment, as well as the alignment of these cases with existing molecular profiles of astrocytic or oligodendroglial tumors in humans. As a result, "gliomatosis cerebri" is no longer considered a distinct tumor entity but rather a type of growth pattern that is rarely found in humans. These tumors are now categorized as either an astrocytoma, oligodendroglioma, or undefined glioma with a diffuse infiltrative growth pattern.4,8
As previously seen with WSC#7 ? Case 1, the tumor classification scheme applied to this neoplasm has changed since its submission to the WSC based on the recommendations from the National Cancer Institute-led multidisciplinary Comparative Brain Tumor Consortium (CBTC). These changes simplify the diagnostic grading scheme of glial tumors and align classification schemes between veterinary and physician neuropathology, which will serve to harmonize future comparative research and hypothesis-based investigations designed to aid in clinical management of both canine and human glioma patients.4
References:
1. Bentley RT, Burcham GN, Heng HG et al. A comparison of clinical, magnetic resonance imaging and pathological findings in dogs with gliomatosis cerebri, focusing on cases with minimal magnetic resonance imaging changes. Vet Comp Onc 2016;14(3):318-30.
2. Higgins RJ, Bollen AW, Dickinson PJ, Siso-Llonch S. Tumors of the Nervous System. In: Meuten DJ ed. Tumors in Domestic Animals, 5th ed. John Wiley and Sons, 2017:839.
3. JPC WSC archives, 2008 Conference 20, Case 1 http://www.askjpc.org/wsco/index.php
4. Koehler JW, Miller AD, Miller CR, et al. A Revised Diagnostic Classification of Canine Glioma: Towards Validation of the Canine Glioma Patient as a Naturally Occurring Preclinical Model for Human Glioma. J Neuropathol Exp Neurol. 2018;77(11):1039-1054.
5. Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. Histological classification of tumors of the nervous system of domestic animals. In: Schulman FY ed. World Health Organization Histological Classification of Tumors of Domestic Animals, Second Series, vol. 5. Armed Forces Institute of Pathology, Washington DC, 1999:p 21.
6. Plattner BL, Kent M, Summers B et al. Gliomatosis cerebri in two dogs. J Am Anim Hosp Assoc 2012; 48:359-365.
7. Porter B, DeLaHunta A, Summers B. Gliomatosis cerebri in 6 dogs. Vet Pathol 2003; 40:97-102.
8. Rissi DR, Donovan TA, Porter BF, Frank C, Miller AD. Canine Gliomatosis Cerebri: Morphologic and Immunohistochemical Characterization Is Supportive of Glial Histogenesis. Vet Pathol. 2021;58(2):293-304.