Signalment:
Gross Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Workers at the University of Florida found this disease to be caused by a retrovirus through the use of transmission electron microscopy. Further characterization efforts conducted demonstrated reverse transcriptase activity. Western blot analysis of viruses from different snakes was also done; these different isolates from different snakes were found to be similar.(1)
Clinical signs are quite variable. Regurgitation and signs of central nervous system (CNS) disease are commonly seen in boa constrictors. Stomatitis, pneumonia, undifferentiated cutaneous sarcomas, and lymphoproliferative disorders have all been seen. Burmese pythons generally show signs of CNS disease without manifesting any other clinical signs; regurgitation is not seen in Burmese pythons.(4)
This snake was one of several in this household that died with similar clinical presentations and pathologic findings. There is no treatment, and the disease is highly contagious and always fatal. As the disease seems to be occurring with increasing incidence, it is imperative that all new boids brought into a collection be quarantined from acquisition for at least 3-6 months, and that appropriate precautions be taken when visiting other collections, pet stores, expositions, and swap events.
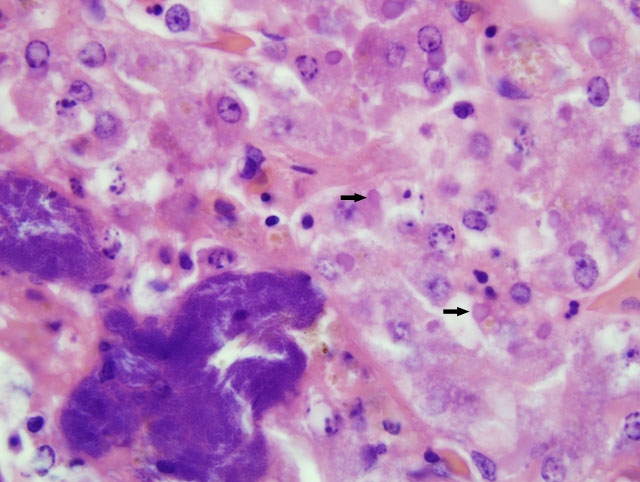
Although slides submitted from this snake for the conference were all from the liver, similar inclusion bodies were also seen in most of the tissues cited above.
JPC Diagnosis:
1. Liver: Hepatocellular degeneration, diffuse, moderate, with scattered single hepatocellular necrosis, and many hepatocellular and biliary epithelial intracytoplasmic eosinophilic inclusion bodies.
2. Liver: Hepatitis, necrotizing, random, acute, multifocal, moderate, with colonies of coccobacilli.
Conference Comment:
Despite an abundance of research on this important entity, fulfillment of Kochs postulates remains elusive, and the cause of inclusion body disease (IBD) is still enigmatic. As noted by the contributor, a retroviral etiology has been strongly suspected, and clinicopathologic evidence (e.g. stomatitis, lymphoproliferative disorders, etc.) supports this conclusion. Moreover, retroviruses have been isolated from various species of snakes with IBD; however, a causal role has not been established, and it remains possible that retroviruses play only an incidental role in IBD. Similarly, reoviruses and adenoviruses have also been isolated from snakes with IBD, and may play causal and/or incidental roles in the disease. Notably, electron microscopy demonstrates that IBD inclusions are nonviral, and consist of radio-dense round particles that accumulate at the periphery of the inclusion; particles are composed of a 68-KDa protein deposited by polyribosomes.(2)
As noted by the contributor, epidemiologic evidence suggests that IBD is indeed contagious, and quarantine measures are therefore warranted. Antemortem diagnosis is achieved by biopsy examination of the liver or kidney, or the esophageal tonsil, in which inclusions are generally present in the overlying epithelial cells.(2) In a recent study, investigators readily identified inclusions in the leukocytes of boas with IBD using concentrated buffy coat examinations, and hence recommended the screening technique as a less invasive alternative to biopsy; however, the technique may not be applicable in pythons, in which the distribution of inclusions is often limited to the central nervous system.(3)
References:
2. Jacobson ER: Viruses and viral diseases of reptiles. In: Infectious Diseases and Pathology of Reptiles, ed. Jacobson ER, pp. 410-412. CRC Press, Boca Raton, FL, 2007
3. Pees M, Schmidt V, Marschang RE, Heckers KO, Krautwald-Junghanns M-E: Prevalence of viral infections in captive collections of boid snakes in Germany. Vet Rec 166:422-425, 2010
4. Schumacher J, Jacobson ER, Homer BL, Gaskin JM: Inclusion body disease in boid snakes. J Zoo Wildl Med 25:511-524, 1994