Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 5
25 August 2019
CASE I: V17-00724 (JPC 4121052).
Signalment: Three years old, female, breed not specified, Ovis aries, sheep
History: The ewe had poor weight gain especially in the winter with periodic loose, nonpelleted feces. The ewe had subcutaneous edema in the skin of the ventral mandibular area (bottle jaw).
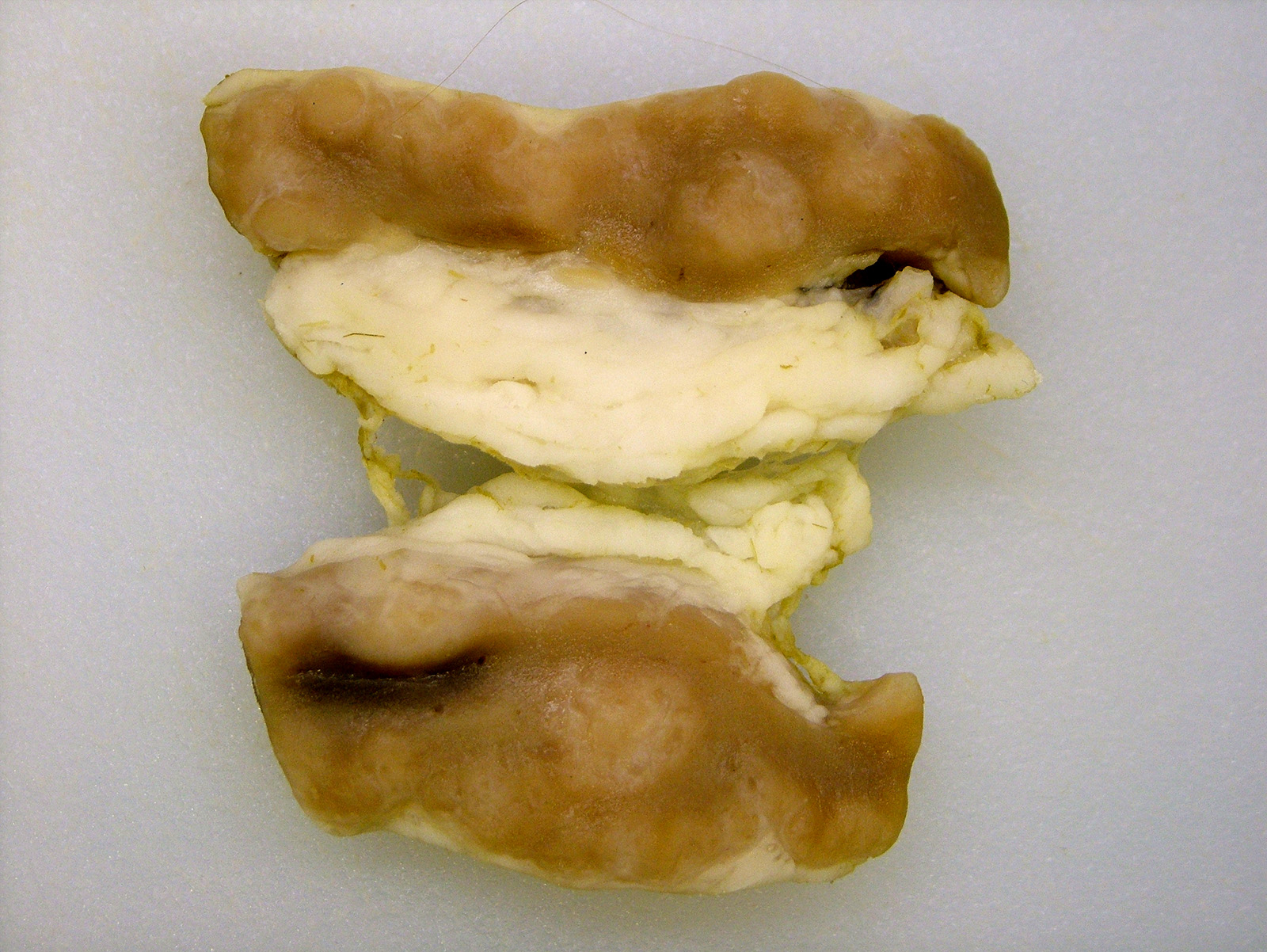
Gross Pathology: There were segments of the small intestine that were thick and corrugated. The mesenteric lymph nodes were enlarged with numerous variably sized tan foci that effaced the lymph node parenchyma.
Laboratory results: The mesenteric lymph node was PCR positive for Mycobacterium avium subspecies paratuberculosis (MAP). MAP was not isolated on culture of the mesenteric lymph node.
Microscopic Description:
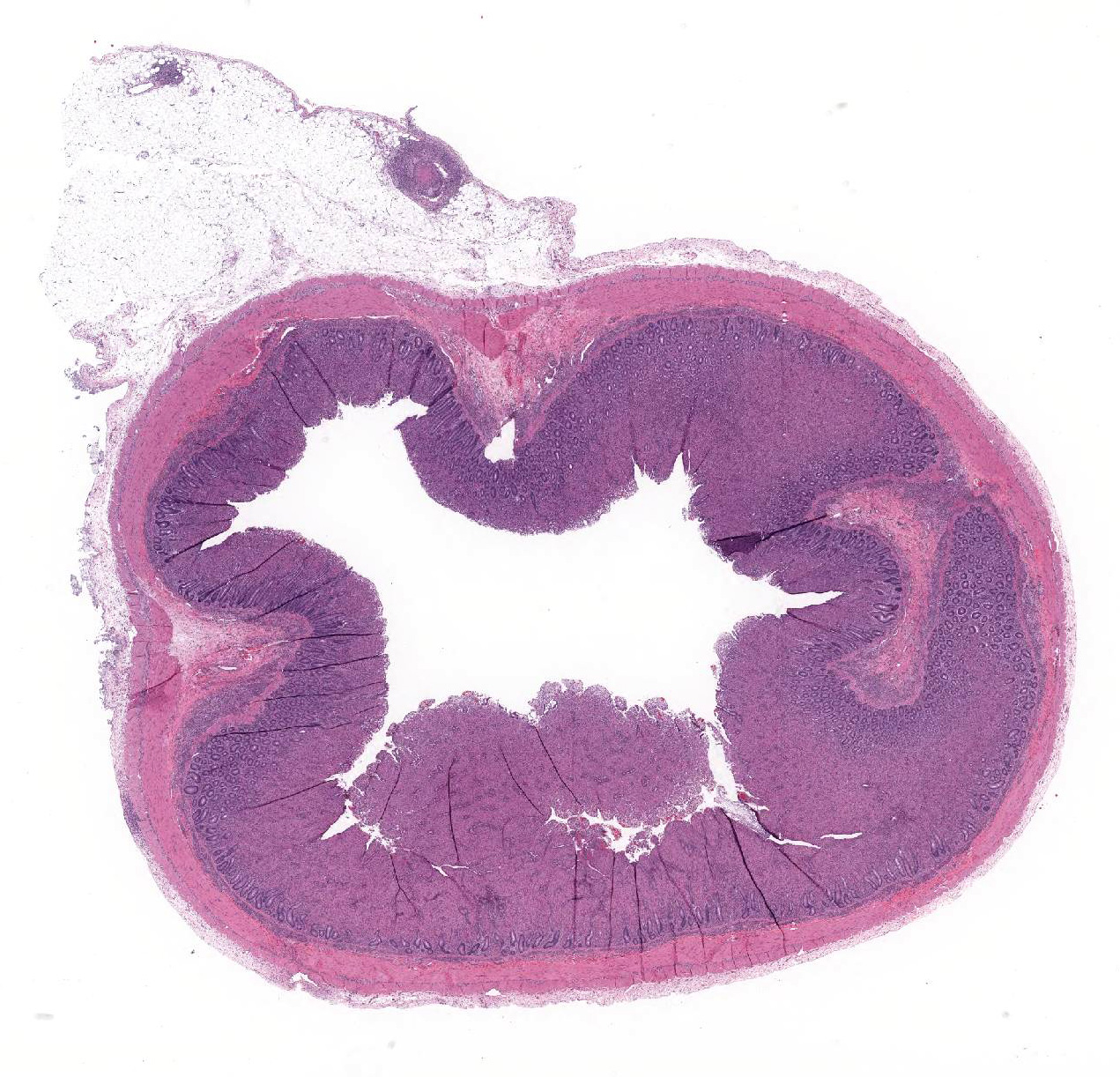
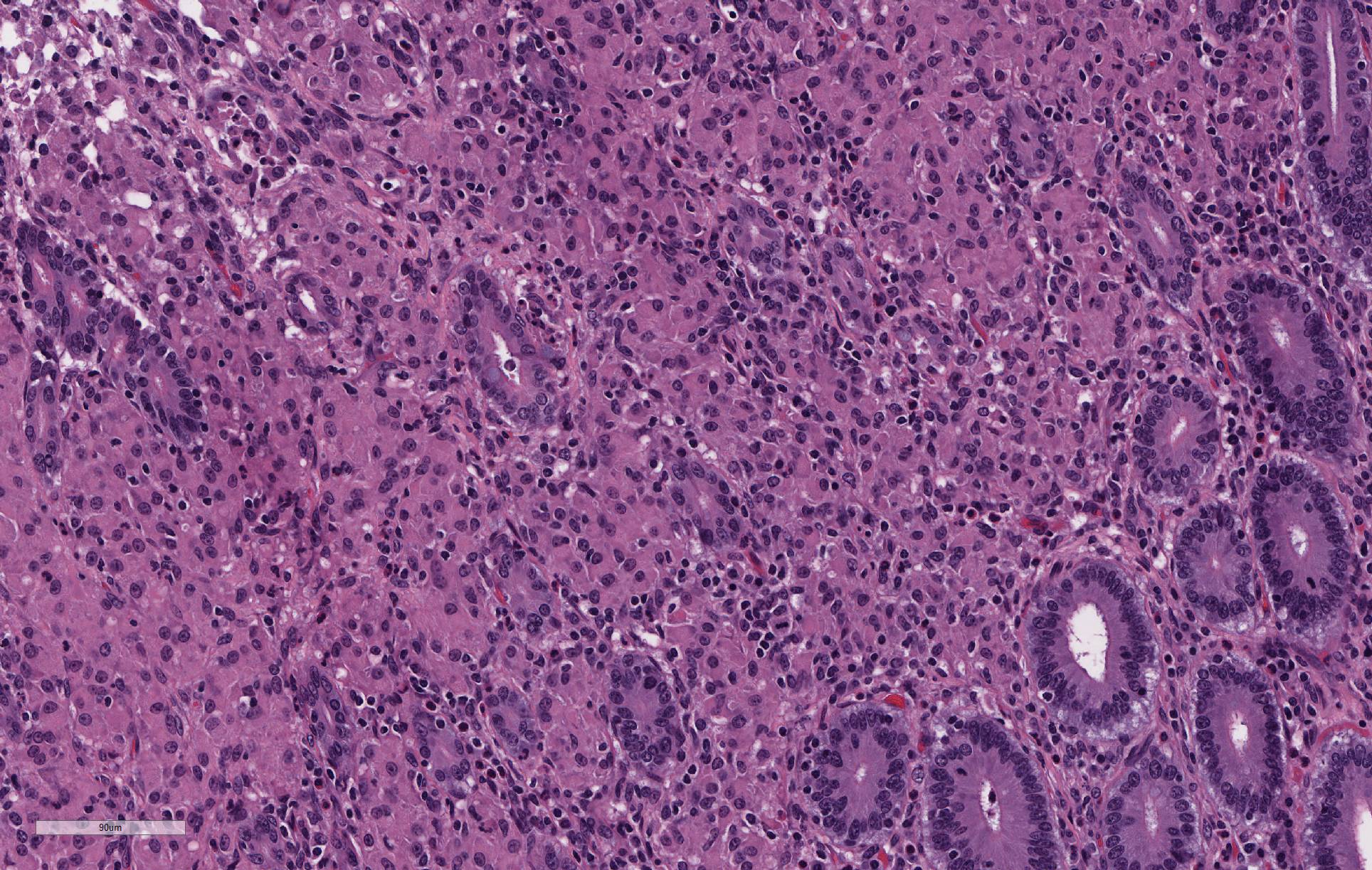
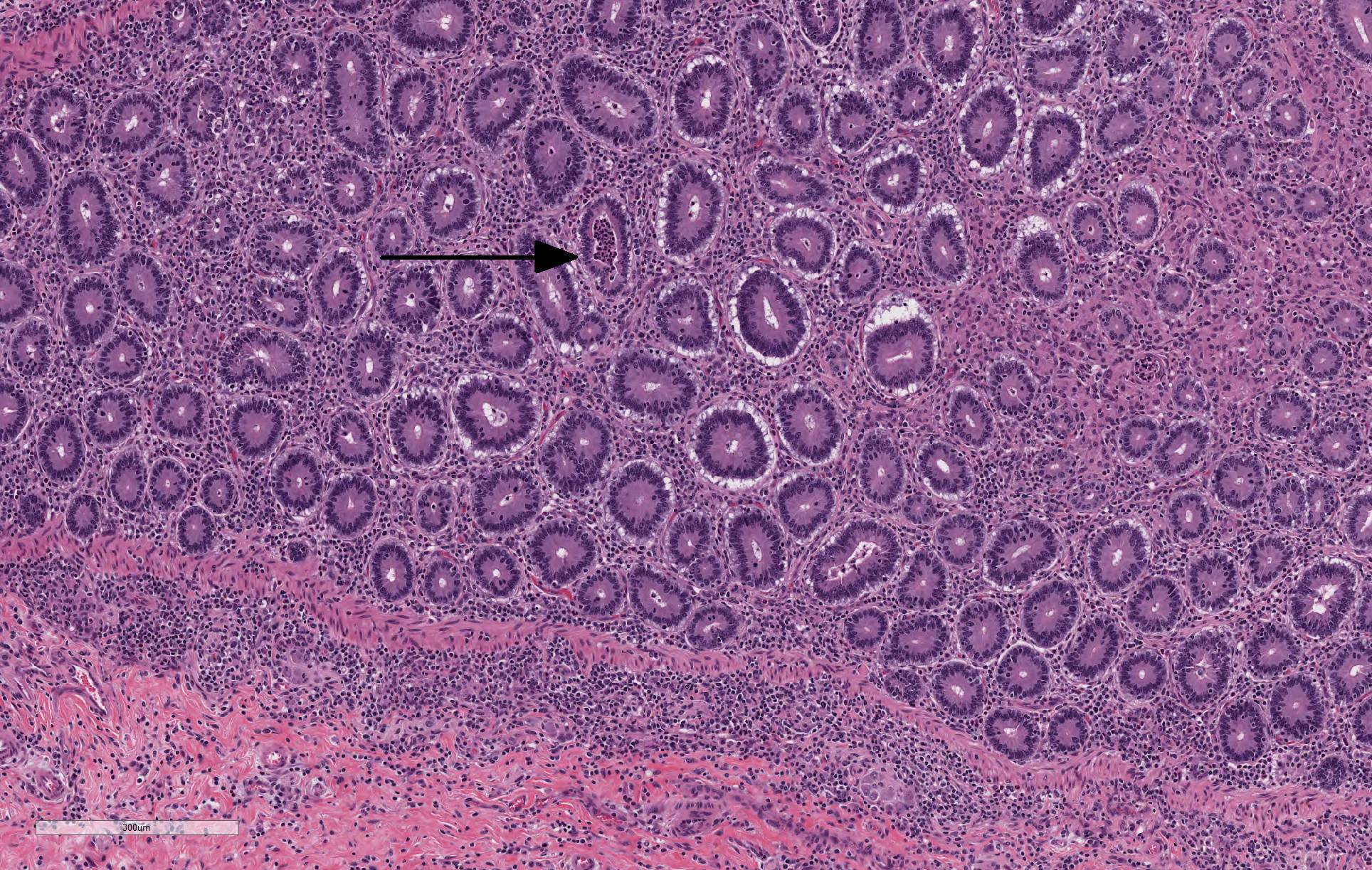
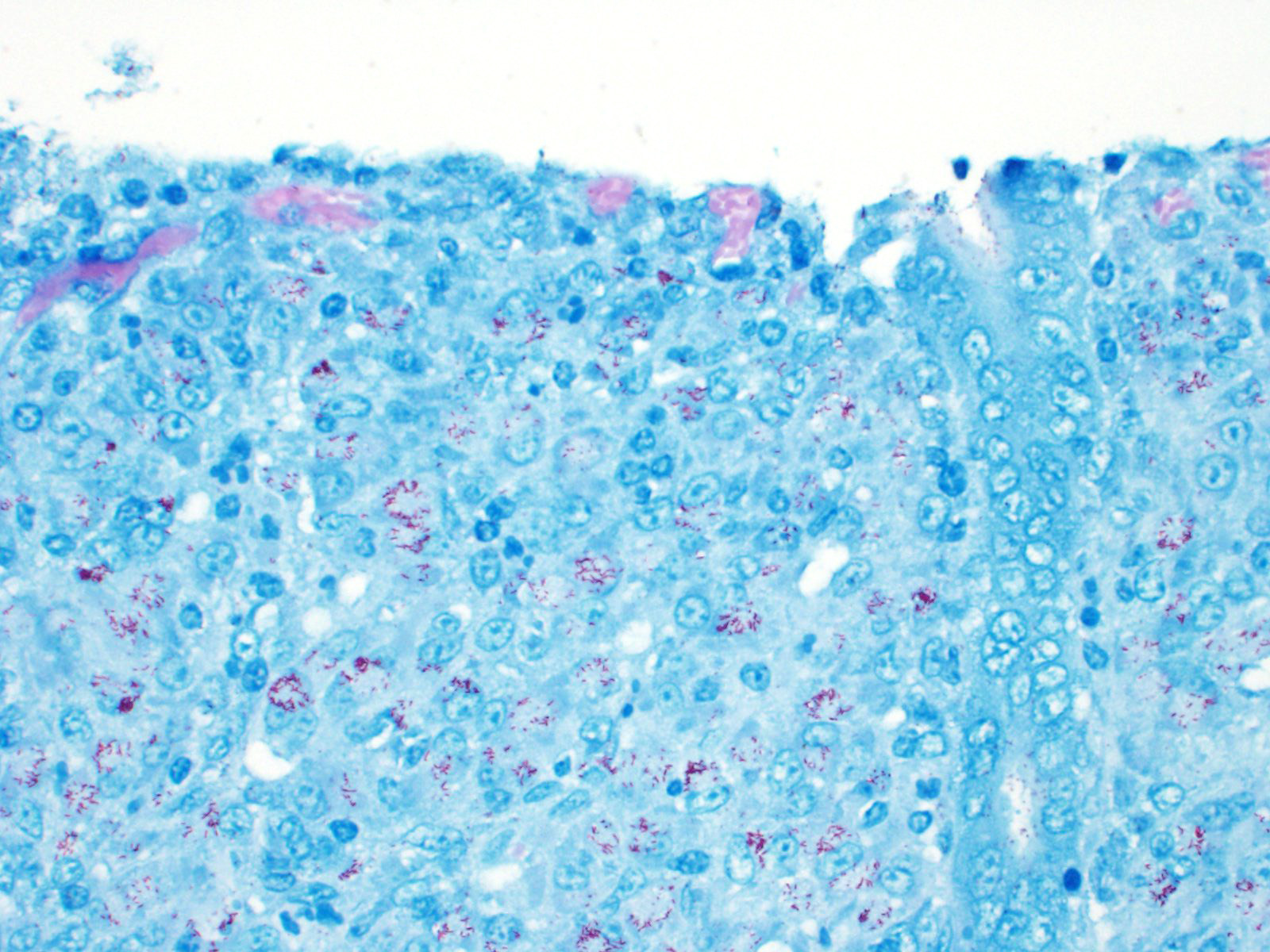
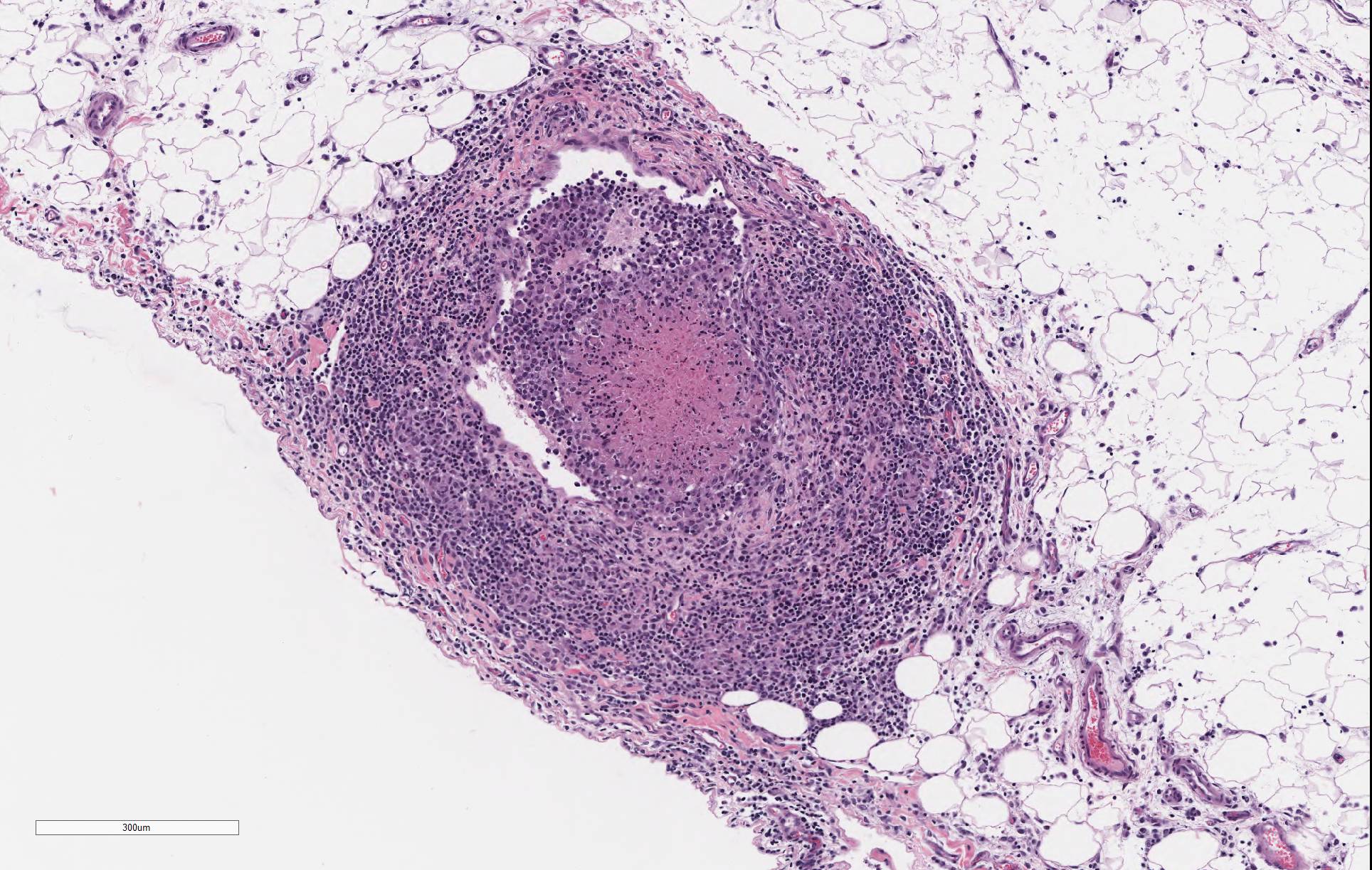
The lamina propria of multiple segments of the small intestine is markedly thickened by diffuse infiltrates of numerous macrophages, which are mixed with lesser numbers of lymphocytes. The infiltrates of macrophages result in blunting and widening of the intestinal villi. The macrophages in the lamina propria contain numerous intracellular acid fast bacilli. There are occasional lymphatic vessels in the mesentery that are surrounded by and infiltrated by numerous macrophages with lesser numbers of lymphocytes and neutrophils (not present in all sections).
Contributor
Morphologic Diagnoses:
Small intestine. Enteritis, granulomatous, diffuse, severe with
numerous intracellular acid fast bacilli; etiology consistent with Mycobacterium
avium subspecies paratuberculosis (Johne’s disease)
Contributor Comment: Mycobacteria are thin rods that can vary in length from 0.2 to 10.0 µm.7 Mycobacteria are acid-fast and are gram-positive, but the lipid in the cell wall typically prevents staining with a Gram stain. They are aerobic, oxidative, non-motile and do not form spores. Mycobacteria are typically slow-growing with a range of generation times of 2-20 hours.
Johne’s disease is caused by Mycobacterium avium subspecies paratuberculosis (MAP).6,7,8 Johne’s disease occurs most commonly in domestic ruminants, but nondomestic ruminant species as well as non-ruminant species can be infected with MAP. MAP is shed in the feces of infected animals and is spread by fecal-oral transmission. Transmission of MAP to naive animals typically occurs in young animals while they are still nursing while older animals are more resistant to infection. There are two strains of MAP: type I or S strain and type II or C strain. The type I strain of MAP was first isolated from sheep and is fairly species specific for sheep with only rare reports of cattle being infected with this strain. The type II strain of MAP was first isolated from cattle and can infect a multitude of species including cattle, sheep and goats. The type II strain of MAP is the most common.
Unlike in cattle where Johne’s disease causes severe diarrhea, Johne’s disease in sheep manifests as a wasting disease in the majority of sheep.3,6,7,8 The intestinal thickening caused by granulomatous enteritis can involve the jejunum, ileum, cecum and colon, but are most common in the ileum.2,3,6,7,8 The intestinal lesions of Johne’s disease in sheep are typically mild, can be multifocal and easily missed at necropsy. Sheep with Johne’s disease can also develop granulomatous lymphangitis of lymphatic vessels in the mesentery as well as lymphadenopathy and granulomatous lymphadenitis of the mesenteric lymph nodes. Tubercle-like caseating mineralized granulomas are more common in the intestine, lymph vessels and mesenteric lymph nodes of sheep than they are in cattle. Lymph nodes other than mesenteric lymph nodes, the liver, the lungs and the spleen can also have small granulomas with MAP in sheep. The microscopic lesions of Johne’s disease in sheep occur in two forms. One form consists of dense infiltrates of numerous macrophages in the mucosal epithelium of the intestine with numerous bacteria (the multibacillary form associated with a strong humoral immune response). The other form consists of focal to multifocal lymphocyte-rich infiltrates of macrophages in the mucosal epithelium of the intestine with few bacteria (the paucibacillary form associated with a cell-mediated immune response).
After ingestion of MAP, the mycobacteria gain access to the small intestine through M cells or epithelial cells over the submucosal Peyer’s patches.2,6,9 The mycobacteria then infect and survive in macrophages in the intestinal mucosal epithelium and the mesenteric lymph nodes. In sheep experimentally infected with MAP, infection could be identified in most sheep by 18 months postinfection.4 Infection was typically identified first in the mesenteric lymph nodes. Microscopic lesions could be identified 6-12 months after the identification of infection with MAP with the lesions usually being identified in the mesenteric lymph nodes first. The microscopic lesions and the disease in the sheep experimentally infected with MAP developed at variable rates with clinical signs first apparent at 24 months usually corresponding to the development of severe intestinal lesions. Although the intestinal lesions may be less severe in subclinical sheep, there is evidence of continuous fecal shedding of MAP in some subclinically infected sheep potentially resulting in a continuously infected environment.10
Diagnosis of Johne’s disease in sheep, particularly of an individual sheep, can be more difficult than it is in cattle.6,8,9 Isolation of mycobacterium from the feces or tissue from sheep is much more difficult than it is from cattle as the type I strain is difficult to isolate.3,6,8,9 PCR can be used to detect MAP in feces and tissue of sheep, but molecular techniques are most likely useful on an individual animal basis and not a herd basis due to the cost of testing.8,9 Serologic testing (ELISA and AGID) can be used for antemortem diagnosis of Johne’s disease, but there are studies that indicate the usefulness of serologic testing depends on whether the sheep has the multibacillary form (a strong humoral immune response and more likely to be serologically positive) or the paucibacillary form (a cell-mediated immune response and more likely to be serologically negative).8 In addition, the serologic tests can also cross react with Corynebacterium pseudotuberculosis (caseous lymphadenitis) another wasting disease of sheep. The diagnosis of Johne’s disease using histopathology collected postmortem or from rectal biopsy can also be difficult with the paucibacillary form of Johne’s disease because it can have a multifocal distribution.4,9
Contributing Institution:
New Mexico Department of Agriculture Veterinary Diagnostic Services
JPC Diagnosis: Small intestine: Enteritis, histiocytic,
diffuse, severe with marked villar and crypt loss, villar blunting and fusion,
crypt abscesses and hyperplasia, and edema (with multifocal lymphohistiocytic
lymphangitis).
JPC Comment: The contributor has provided a concise review of Mycobacterium avium susbsp. paratuberculosis (MAP) in ruminants with a focus on its peculiarities in sheep.
The economic importance of Johne’s disease in sheep cannot be ignored. While reports in small ruminants, as compared to affected cattle are sketchy, annual mortality in affected flocks in Australia have averaged 6-7% annually, with some reaching up to 20%. Economic losses to the British sheep industry range up to 20 million pounds annually if replacement strategies are factored in. In Italy, MAP infection decreases profit efficiency by 20% in affected farms. 10 These are direct losses only; potential indirect losses arising from trade restrictions at the international and national levels are difficult to estimate. Unlike many other highly contagious diseases of serious economic import, the OIE as of yet considers this a non-reportable disease and offers no guidance on paratuberculosis. Norway has had no known cases of MAP since 2015, and Sweden has been free of Johne’s disease in cattle since 2008, but due to the insidious nature of this disease, all countries remain at risk.10 Another risk factor is Johne’s disease infection of non-domestic ruminant species including wild species of goats and sheep, deer, camelids, bison, rhinoceroses, and some species of marsupials may serve as additional reservoirs.
Another determining factor in the potential spread of Johne’s disease appears to be the differential susceptibility of various breeds to infection by MAP. In a recent article by Begg at al.,1 Merino sheep (prized for their fine wool with an average value of 2-3 times more than mutton sheep) have the highest rate of clinical disease 14 months after oral inoculation at 42%, followed by Merino-Suffolk cross (36%), Border Leicester (12%) and Poll Dorset (11%). The authors were careful to point out that this may simply imply a longer disease duration Border Leicester and Poll Dorset breeds; however, this information is useful when considering control programs that emphasize a rapid decrease in environmental contamination by infected animals.1
Over the years, the MAP has been considered as a potential pathogen in humans in a number of conditions, yet never definitively incriminated. Numerous studies have identified potential risks for both waterborne (farm effluent) and foodborne (contaminated milk and meat) pathways for zoonotic transmission to humans, however at present, MAP is still not considered a zoonotic pathogen. One of the most common theories is that MAP may be a cause of IBD in humans, and is supported by circumstantial evidence including similar morphologies between Johne’s disease and various forms of IBD including Crohn’s disease, increases in IBD in areas in which animals demonstrate a 30% incidence of Johne’s disease and isolation in breast milk from mothers with IBD. However, Koch’s postulated have never been fulfilled, and MAP has not yet been isolated with by ELISA-based or PCR in individuals with IBD. MAP has also been theorized to play a role in the generation of Type I diabetes in certain populations due to potential epitope mimicry between islet cell proteins GAD65 and ZNT8 with MAP proteins and HSP65 and MAP3865c,, respectively as well as and immune responses to HSP65 have been seen in patients with rheumatoid arthritis and Hashimoto’s thyroiditis. 4
The moderator discussed the
difference between Ziehl-Nielsen and Fite-Furaco stains. â"Acid- fastness" refers to microorganisms
whose cell wall has a high lipid content of mycolic and long chain fatty acids,
which cause them to bind the basic dye carbol-fuchsin, a stain which remains after
strong decolorization with acid-alcohol (thus "acid-fast") The
Fite-Furaco uses peanut oil and xylene to protect the wall of bacteria from
decolorization and allows for the visualization of bacteria with less lipid
content, such as M. leprae and Rhodococcus equi. The moderator
also mentioned a newly discovered strain of the bacterium, called the type III
B strain (as opposed to type I S and type II C strains) which is pathogenic for
buffalo, and currently a problem in India.
References:
1. Boes KM and Durham AC. Bone marrow, blood cells and the lymphoid lymphatic system. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO. Elsevier; 2017: 724-804.
2. Carrigan MJ and Seaman JT. The pathology of Johne’s disease in sheep. Aust Vet J. 1990; 67(2): 47-50.
3. Dennis MM, Reddacliff LA and Whittington RJ. Longitudinal study of clinicopathological features of Johne’s disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis. Vet Pathol. 2011; 48(3): 565-575.
4. Garvey M. Mycobacterium avium subspecies paratuberculosis: A possible causative agent in human morbidity and risk to public health safety. Open Vet J 2018; 8(2):172-181
5. Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO. Elsevier; 2017: 324-411.
6. Markey B, Leonard F, Archambault HM, Cullinare A and Maguire D. Mycobacterial species. In: Clinical Veterinary Microbiology. 2nd ed. St. Louis, MO. Mosby Elsevier; 2013: 161-176.
7. Radostits OM, Gay CC, Hinchcliff KW and Constable PD. Diseases associated with bacteria â IV. In: Veterinary Medicine A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 10th ed. Philadelphia, PA; 2007: 1007-1060.
8. Uzal FA, Plattner BL and Hostetter Jm. Alimentary system. In: Maxie G, ed. Jubb, Kennedy, and Palmerâs Pathology of Domestic Animals. 6th ed (Vol 2). St. Louis, MO. Elsevier; 2016: 1-257.
9. Whittington RJ, Reddacliff LA, March I, McAllister S and Saunders V. Temporal patterns and quantification of excretion of Mycobacterium avium subspecies paratuberculosis in sheep with Johne’s disease. Aust Vet J. 2000; 78(1): 34-37.
10. Whittington R, Dopnat K, Weber MF et al. Tontrol of paratuberculosis: who, why, and how. A review of 48 countries. BMC Vet Res 2019; 15:198-227.