Signalment:
Gross Description:
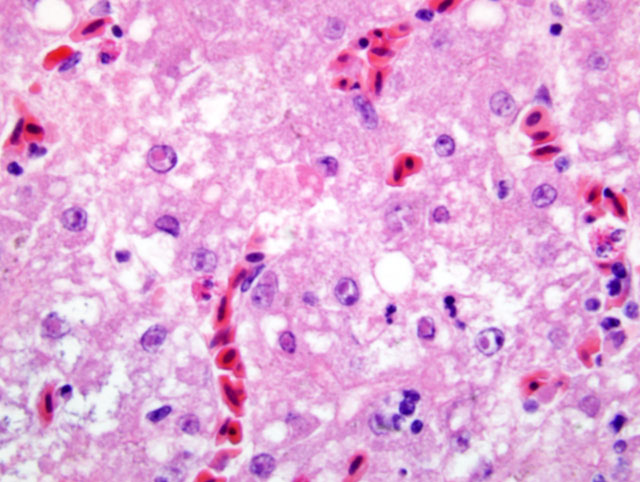
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In this bird, in addition to the liver lesions, mucosal lesions were noted in the digestive tract (esophagus, proventriculus), characterized by erosions and ulcerations with rare eosinophilic intranuclear inclusion bodies in few remaining epithelial cells. Typically, lesions of DVE are those of vascular damage, eruptions of specific locations on mucosal surface of the gastrointestinal tract, lesions of lymphoid organs, and degenerative sequelae in parenchymatous organs. When these lesions are collectively present, are diagnostic of DVE.(6) In the present case the diagnosis was confirmed by PCR on tissue pools.Â
Differential diagnosis requires consideration of other diseases producing hemorrhagic and necrotic lesions in anseriforms. Such diseases include duck virus hepatitis, pateurellosis, necrotic enteritis, coccidiosis, and specific intoxications.(6)
To prevent this disease from spreading, strict decontamination and depopulation should be carried out whenever possible.(3) A vaccine is also available for prevention of DVE but approval by animal health authorities is required before it can be used.(7)
JPC Diagnosis:
Conference Comment:
The pathogenicity of this virus depends on the particular species of duck infected. The blue-winged teal (Anas discors) is most susceptible to DVE, while the pintail duck (Anser acuta) is the least susceptible.(2) Muscovy ducks, as seen in this case, are highly susceptible to this particular virus. This virus is a major concern because migratory birds can spread this virus to na+�-�ve populations leading to massive outbreaks with high mortality.(4)
Dr. Raymond briefly integrated some general pathology topics as they relate to this particular case, and he focused on mechanisms by which free radicals cause damage to cells.
Free radicals are molecules with an unpaired electron making them highly reactive. These molecules are generated as by-products of normal cell function via oxidative metabolism or are a by-product of exposure to radiation, toxins, drugs, or other chemicals. Free radicals are also produced by neutrophils and macrophages in inflammation. Free radicals can damage cells by 1) peroxidation of cellular phospholipid membranes; 2) DNA injury; 3) oxidative modification of proteins.(1)
Free radical damage is minimized by several different antioxidants within the body. These antioxidants include enzymatic and nonenzymatic antioxidants. Enzymatic antioxidants include superoxide dismutase, catalase, and glutathione peroxidase. Nonenzymatic antioxidants include transport proteins transferrin, ferritin, lactoferrin, and ceruloplasmin. Vitamins A, C and E, and other dietary compounds such as lycopenes, flavonoids, genistein, and reserpines help to minimize the damage done by free radicals.(1)
References:
2. Burges, EC, Ossa CJ, Yuill TM: Duck Plague: a carrier state in waterfowl. Avian Dis 23:940-949. 1979
3. Davidson S, Converse KA, Hamir NA, Eckroade RJ: Duck viral enteritis in domestic muscovy ducks in Pennsylvania. Avian Dis 37:1142-1146, 1993
4. Leibovitz L, Hwang J: Duck plague on the American continent. Avian Dis 12:361-378, 1967
5. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ: Herpesviridae. In: Veterinary Virology, eds. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ, 3rd ed., pp. 301-325. Academic Press, San Diego, California, 1999
6. Sandhu, TS, and Leibovitz L: Duck virus enteritis (duck plague). In: Disease of Poultry, eds., Calneck BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, 10th ed., pp. 675-682. Iowa State University Press, Ames, IA, 1997
7. Whiteman CE, Bickford AA: Avian disease manual. 3rd ed. American Association of Avian Pathologists, Kennett Square, PA, 1988
8. Kumar V, Abbas AK, Fausto N: Robbins and Cotran, Pathologic Basis of Disease, 7th ed., pp. 16-18. Elsevier Saunders, Philadelphia, PA, 2005