Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 19
30 January, 2019
CASE III: 15-41009 (JPC 4084545).
Signalment: Adult, intact male tiger (Panthera tigris)
History: The animal presented for necropsy examination following euthanasia after a 3-week history of progressive pneumonia that was unresponsive to antibiotic administration.
Gross Pathology: The lungs were mottled pink to red and rubbery to firm on palpation. The right middle lung lobe was more meaty and consolidated than the other lobes. There were also multiple pale tan, round, variably sized up to 2 cm in diameter nodules interspersed throughout the pulmonary parenchyma. The bronchi within the cranioventral lungs were often filled with frothy and purulent material.
Laboratory results: Virology: The lungs were positive with fluorescent antibody testing for Canine Distemper Virus. Virus isolation was unsuccessful.
Bacteriology:
PCR on lung tissue for Mycoplasma sp. was negative.
Aerobic Culture and identification using MALDI-TOF -
| Specimen | Isolate | Level |
| Lung | Acinetobacter sp. | few |
| *Result Comment: | ||
| Identified by MALDI-TOF with a score as A. haemolyticus of 2.331. | ||
| Lung | Escherichia coli | v rare |
| Lung | Ochrobactrum | v few |
| Lung | Pseudomonas pseudoalcaligenes | few |
| Lung | Gram-negative rod | v few |
| *Result Comment: | ||
| Identified as Rhizobium radiobacter by MALDI-TOF with a score of 2.308. | ||
| Lung | Alcaligenes faecalis | v rare |
*General Test Comment:
MALDI-TOF Score:
Range Description
2.300…3.000 Highly probable species identification
2.000…2.299 Secure genus identification, probable species identification
1.700…1.999 Probable genus identification
0.000…1.699 Not reliable identification
Microscopic Description:
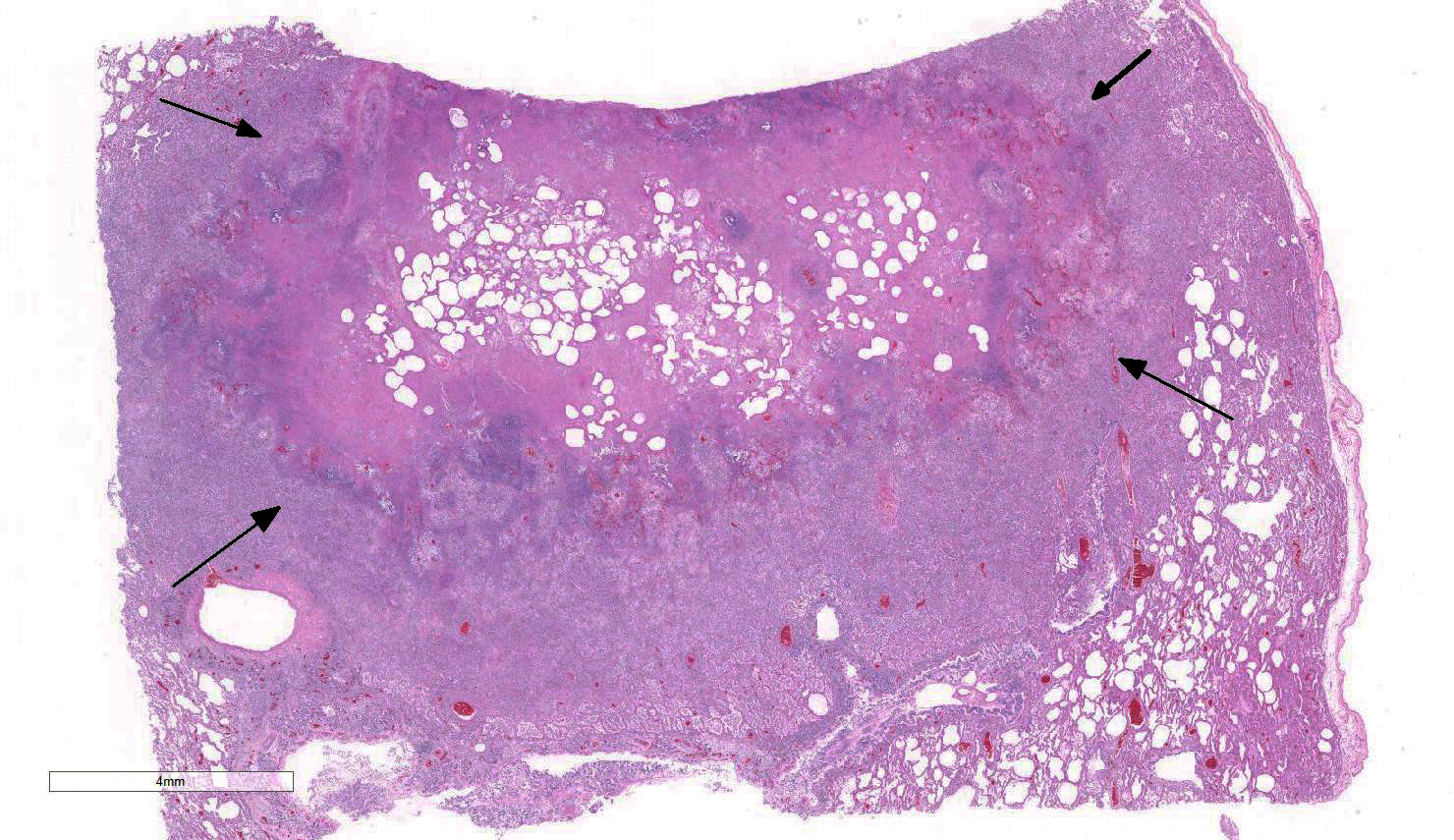
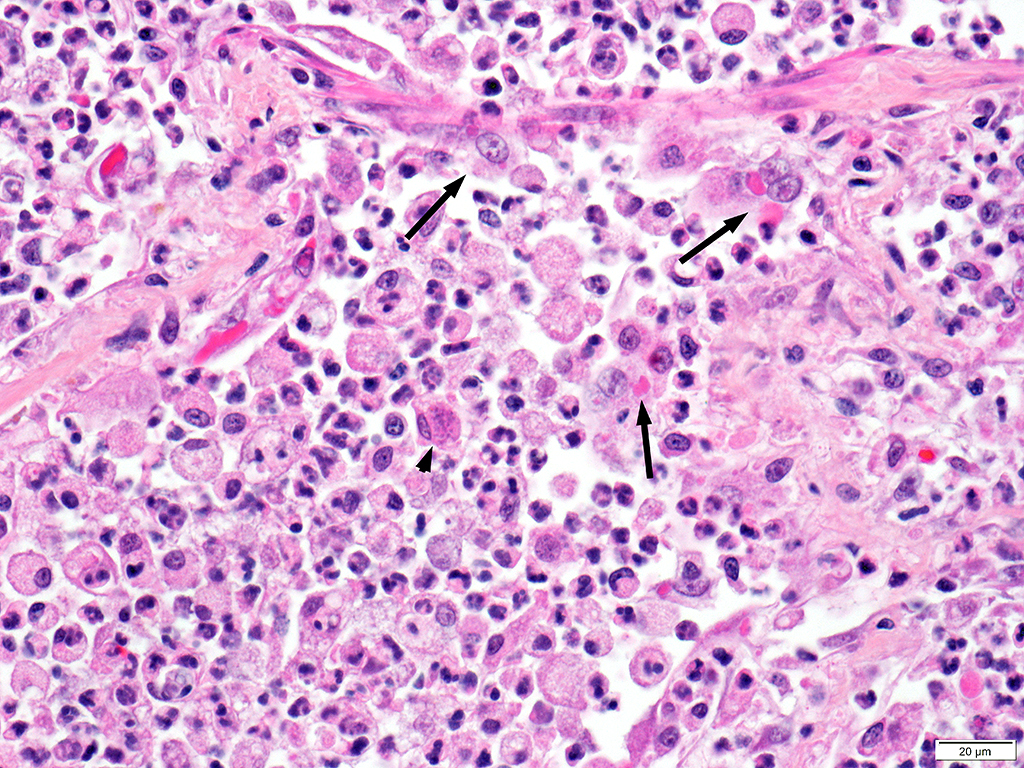
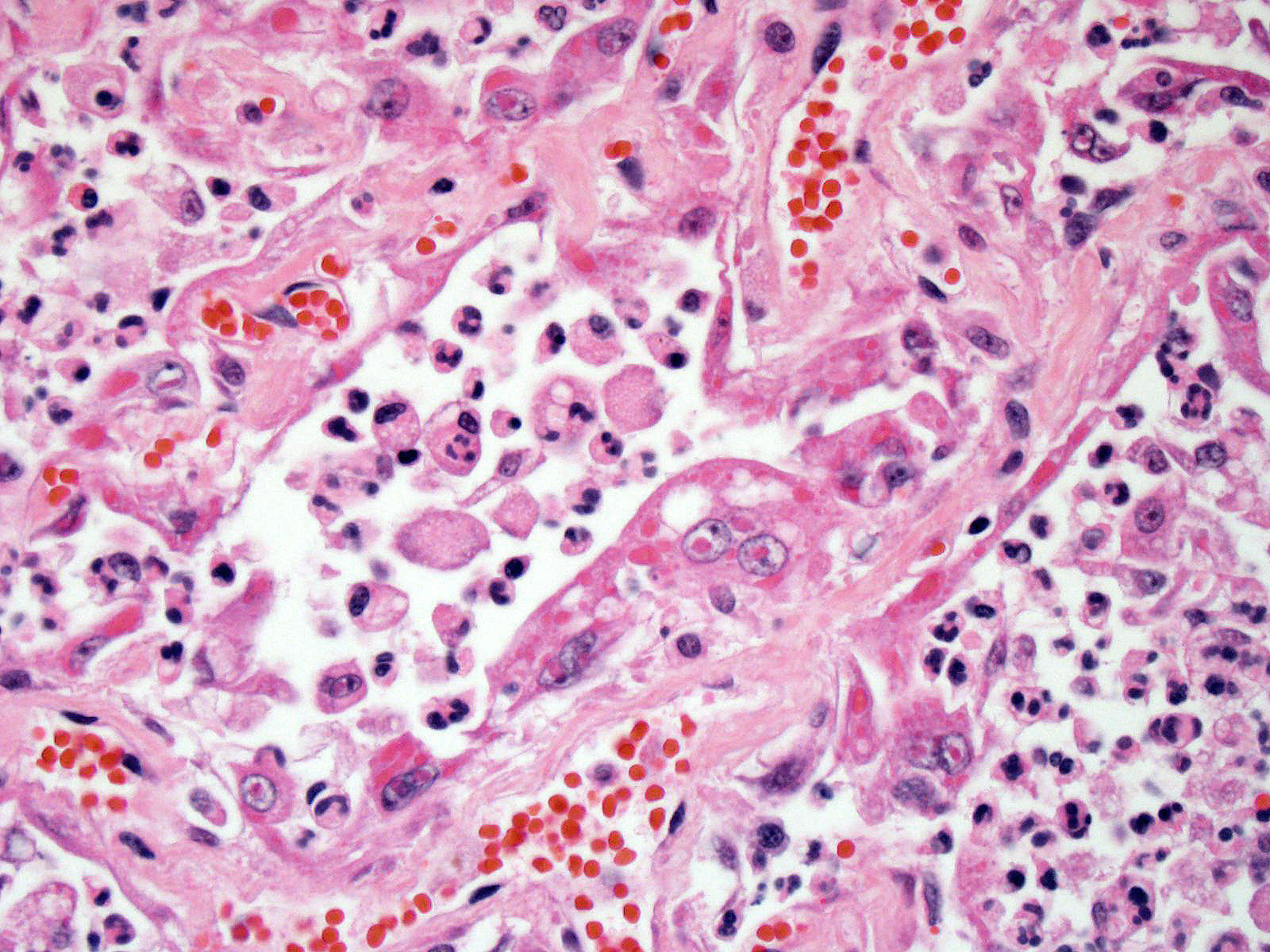
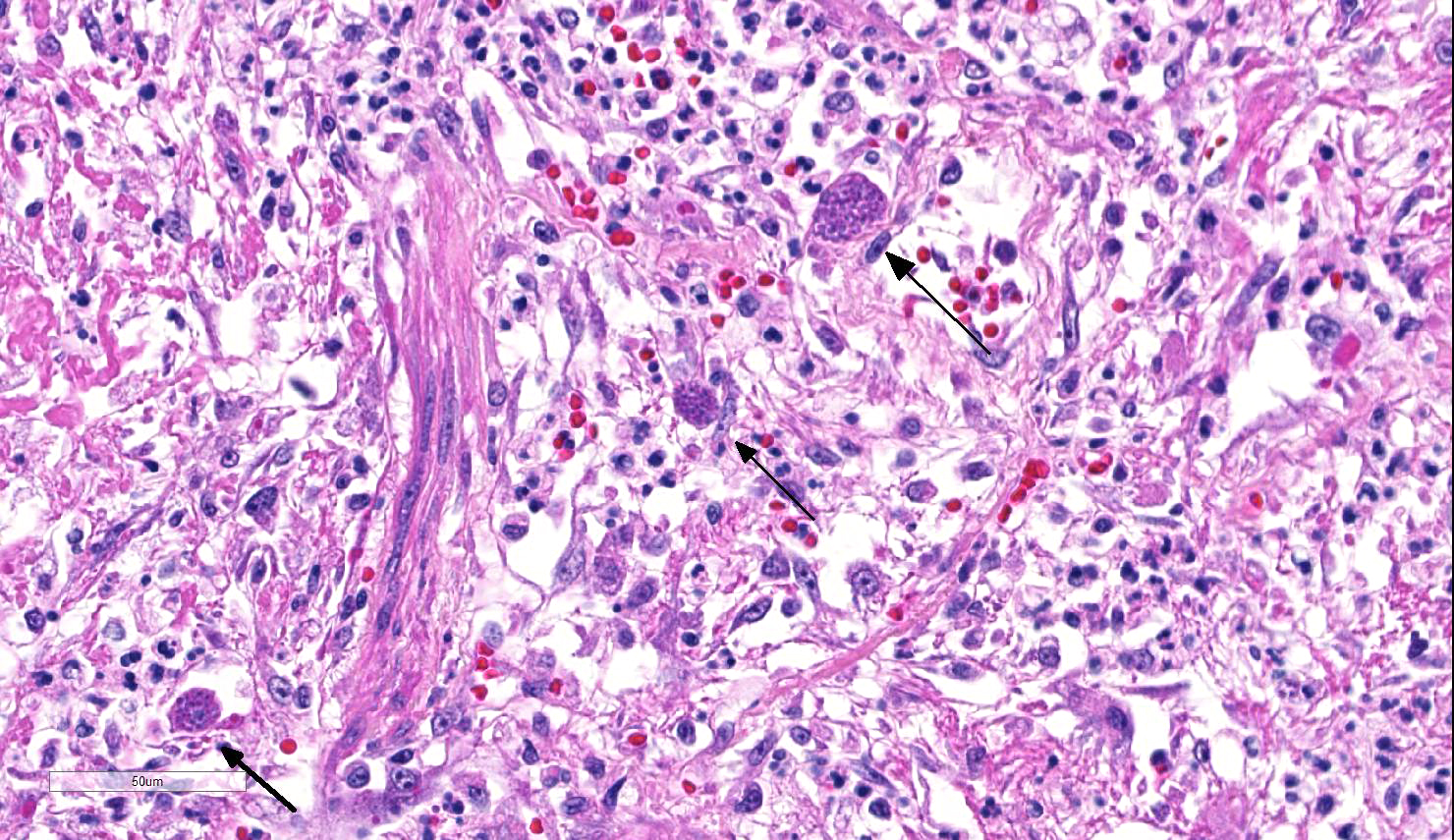
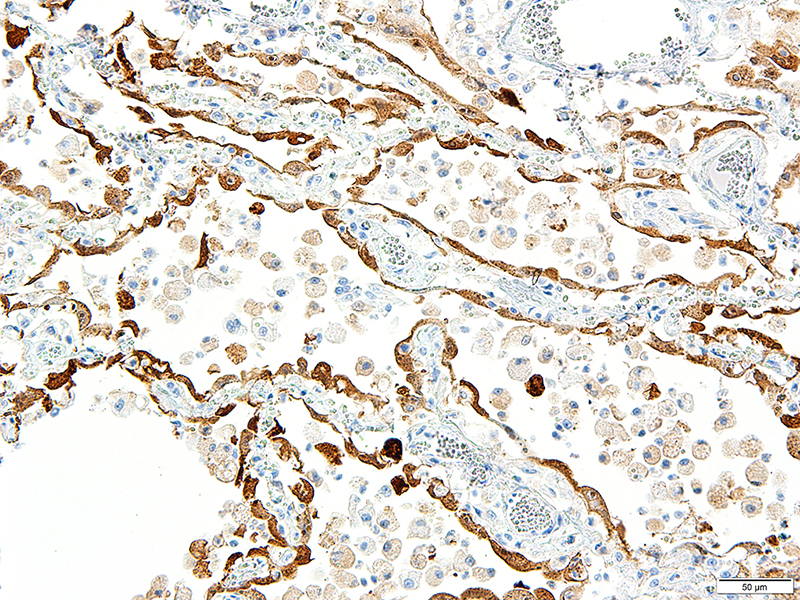
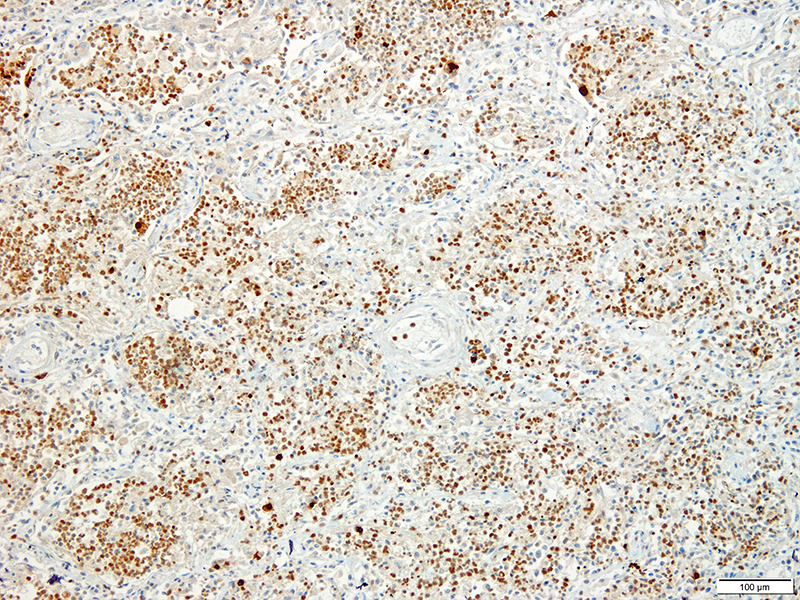
Lung: Approximately 80% of the section is affected by a profound inflammatory process. Alveolar spaces are frequently filled with many viable and degenerate neutrophils mixed with foamy macrophages, abundant eosinophilic, fibrillar material (fibrin), moderate cellular debris, and mild hemorrhage. The alveolar septa are diffusely thickened up to 5 times their normal diameter by moderate numbers of similar inflammatory cells, marked type II pneumocyte hyperplasia, and moderate smooth muscle hypertrophy. Pneumocytes often form multi-nucleated syncytial cells that contain up to 7 nuclei. Many pneumocytes and syncytial cells contain one to several variably sized and shaped, eosinophilic, glassy, intracytoplasmic and intranuclear inclusion bodies. There is multifocal, extensive necrosis with abundant pyknotic, karyorrhectic, and karyolytic cellular debris, and alveoli, bronchioles and bronchi in these areas are frequently filled with innumerable degenerate neutrophils mixed with fewer macrophages and eosinophils. Many macrophages are enlarged up to 70 µm in diameter and contain an intracytoplasmic, 20 x 15 µm protozoal cysts that are filled with small, 1-2 µm bradyzoites, as well as non-encysted and similarly sized tachyzoites. Cysts and tachyzoites are also present free within the extracellular space.
Contributor’s Morphologic Diagnoses:
Lung: Diffuse, marked, fibrinonecrotic, suppurative, histiocytic, bronchointerstitial pneumonia with intracytoplasmic and intranuclear epithelial inclusions (Canine Distemper Virus) and protozoal cysts and free zoites (Toxoplasma gondii).
Contributor’s Comment: Canine distemper virus (CDV) inclusions were also identified within the glandular epithelium of the gastric mucosa in this case. There were no identified abnormalities within the nervous system. Toxoplasma gondii organisms were also found within a tracheobronchial lymph node. Additional findings in this case included multiple mast cell tumors within the liver and spleen. This animal was part of a larger outbreak of CDV in a group of big cats.
CDV is in the family Paramyxoviridae, subfamily Paramyxovirinae, genus Morbillivirus. It is a 100–700 nm, irregularly shaped virus composed of an outer lipoprotein envelope, and inner matrix, and a nucleocapsid that contains single-stranded negative-sense RNA Infection occurs by inhaling aerosols or close contact with infected animals. The virus enters via the epithelium and infects upper respiratory tract macrophages which transport it to local lymphoid tissues where the virus replicates further, and spread continues unless adequate cell-mediated and humoral responses are mounted.7,11 Clinical signs in CDV infection most often include the respiratory, nervous, and gastrointestinal systems. Many other tissues may be affected as well. Key histologic changes in CDV infection are eosinophilic, homogenous or glassy, variably sized intranuclear and intracytoplasmic inclusion bodies, which are most easily identified in the nervous system and epithelial tissues. In the lungs, commonly observed changes include bronchointerstitial pneumonia accompanied by viral inclusions within airway epithelium, epithelial syncytial cell formation, and type II pneumocyte hyperplasia.7
The host range of CDV is wide and continuing to grow. A recent retrospective review5 compiled all reported natural and experimental CDV infections. Susceptible animals include those from Carnivora (Canidae, Felidae, Mustelidae, Procyonidae, Hyaenidae, Ursidae, Phocidae, Viverridae, Ailuridae, Mephitidae, Odobenidae, and Otariidae), Rodentia (Muridae, Cricetidae, Sciuridae, and Caviidae), Primates (Cercopithecidae and Cebidae), Artiodactyla (Suidae, Tayassuidae, and Cervidae), and Proboscidea (Elephantidae) are susceptible to CDV.5
Toxoplasma gondii is a heteroxenous apicomplexan protozoan parasite that is capable of infecting virtually all homeothermic animals. It is an obligate intracellular parasite. Felids serve as definitive hosts, while other animals are intermediate hosts. Many if not all cells and tissues are susceptible to infection. Infection of non-intestinal tissues is achieved by initial ingestion of oocysts, excysting of sporozoites followed by their penetration of small intestinal mucosal and lamina propria cells, and replication within these small intestinal cells. With continued replication and formation of tachyzoites, cells eventually rupture, and tachyzoites infect new cells and are capable of spreading to distant tissues via blood or lymph.4,6 Necrosis predominates in gross lesions observed and typically presents as variably sized gray foci within any tissue. Within the lung, microscopic changes closely follow that of interstitial pneumonia. Alveolar septa are initially thickened by mononuclear cells with fewer granulocytes, followed closely by type II pneumocyte hyperplasia. Alveolar lumina are filled with macrophages and fibrin. Necrosis is abundant with continued parasitic replication. Intra- or extracellular cysts and zoites within affected areas are frequently observed and are highly suggestive of toxoplasma infection.4,6
Infection is believed to be widespread among humans, up to 80% in some parts of the world.(7) Seroprevalence is high in several studies involving zoo and wild animals, up to 90% in wild animals (foxes), and 52.4%, 64.5%, and 81.4% in zoo felids.1,3,9,10 Toxoplasma gondii has high zoonotic potential, and though high seroprevalence with little clinical disease indicates that most infections are asymptomatic, infection in immunocompromised individuals can cause severe illness and often death. With the current case, the tiger was undoubtedly immunocompromised from concurrent CDV infection.
Contributing Institution:
University of Illinois College of Veterinary Medicine
Department of Pathobiology
2522 Veterinary Medicine Basic Science Building
2001 S. Lincoln Ave.
Urbana, IL
61802
http://vetmed.illinois.edu/research/departments/pathobiology/
JPC Diagnosis: Lung: Pneumonia, bronchointerstitial (1pt.), necrotizing (1pt.) and fibrinosuppurative (1pt.), diffuse, severe, with numerous intraepithelial intracytoplasmic and intranuclear viral inclusions (1pt.), viral syncytia and intraepithelial and intrahistiocytic apicomplexan zoites. (1pt.)
JPC Comment: Transpecies infection with canine morbillivirus is not a new phenomenon. Canine morbillivirus infection (CDV) in non-canine host as first reported in silver jackals in 1937 in South Africa; the first cross-species infection in the U.S. (the country with the largest number of confirmed reports occurred in a badger in Colorado in 1942.5 Inter-Order infection was first accomplished experimentally in hamsters in the early 1960s (but required cerebral inoculation.)5 Natural intra-order infection occurred in the late 1970s and 1980s in primates. While transspecies infection by CDV (and high mortality) is primarily in carnivore species (prompting at least one call to rename it “Carnivore Distemper Virus”,12 herbivorous species such as collared peccaries and non-human primates are documented as well.2 Outbreaks of morbillivirus disease in seals may result from either CDV or closely related phocine distemper virus. Dolphins, harbor porpoises and pilot whales all have genetically different morbillivirus which cause similar disease (and have been transmitted to Mediterranean monk seals, but interestingly, their morbillivirus appears more closely related to the morbillivirus causing pestis du petits in ruminants and that which caused rinderpest.2
Rarely associated with non-human primates, CDV infection has occurred in a number of species of macaques in Japan and China since the first case occurred in Japanese macaques in 1989.8 CDV was responsible for up to 4000 rhesus fatalities in a breeding program in China, in which animals displayed measles-like symptoms with respiratory distress, rashes over the entire body, reddening and swelling of the footpads, conjunctivitis, and a thick nasal discharge. Interstitial fibrosis was a predominant finding at autopsy. Following sequencing of the viral genome, it showed 97% homology with other CDV isolates from China. A source for this and related outbreaks was not identified.8
Molecular evolutionary analysis of CDV has suggested that mutations in the signaling lymphocyte activation molecule (SLAM, CD150 - the common receptor used by morbillivirus to gain entry into the host cell - and the CDV hemagglutinin (HA) protein that binds the SLAM molecule are important in cross-species infection as well as species specificity. 2,12 An area of particular concern in the future would be the potential for CDV strains that infect primates, which already are able to use the human nectin-4 receptor for cellular entry, to mutate in the region of the viral H protein and become infective for humans. 2
The moderator reviewed the history and epidemiology of morbillivirus infection in since the establishment of the veterinary school in Lyon, France in 1762 (in order to combat periodic outbreaks of rinderpest in Europe.)
References:
1. Alvarado-Esquivel C et al. Seroprevalence of toxoplasma gondii infection in captive mammals in three zoos in Mexico City, Mexico. Journal of Zoo and Wildlife Medicine. 2013;44(3):803-806.
2. Beineke A, Baumgartner W, Wohlsein P. Cross-species transmission of canine distemper virus-an update. One Health 2015; 1:49-59.
3. de Camps S et al. Seroepidemiology of toxoplasma gondii in zoo animals in selected zoos in the Midwestern United States. Journal of Parasitology. 2008;94(3):648-653.
4. Dubey JP and Lappin R. Toxoplasmosis and Neosporosis, In: Green CE ed.
Infectious diseases of the dog and cat, 3rd ed. St. Louis, MO. Elselvier: 2006:754-768.
5. Martinez-Gutierrez M, Ruiz-Saenz J. Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Vet Res. 2016;12:78.
6. Maxie, MG. Toxoplasmosis, In: Maxie, MG ed. Jubb, Kennedy, and Palmer's pathology of domestic animals, 6th ed., Vol. 2. St. Louis, MO. Elselvier; 2016:236-237.
7. Maxie, MG. Canine distemper, In: Maxie, MG ed. Jubb, Kennedy, and Palmer's pathology of domestic animals, 6th ed., Vol. 2. St. Louis, MO. Elselvier; 2016:574-576.
8. Qui W, Zheng Y, Zhang S, Fan Q, Liu H, Zhang F, Wang W, Liao G, Hu R. Canine distemper outbreak in rhesus monkeys, China. Emerg Inf Dis 2011; 8:1541-1543.
9. Samuel WM, Pybus MJ, and Kocan AA. Toxoplasmosis and related infections, In: Samuel WM, Pybus MJ, and Kocan AA eds. Parasitic diseases of wild mammals, 2nd ed. Ames, IA: Iowa State Press; 2001:478-519.
10. Silva JCR et al. Toxoplasma gondii antibodies in exotic wild felids from Brazilian zoos. Journal of Zoo and Wildlife Medicine. 2001;32(3):349-351.
11. Terio KA, Craft ME. 2013. Canine distemper virus (CDV) in another big cat: should CDV be renamed carnivore distemper virus? mBio 4(5):e00702-13. doi:10.1128/mBio.00702-13.
12. Williams, ES. Canine distemper, In: Williams ES, Barker IK, eds. Infectious diseases of wild mammals, 3rd ed. Ames, IA: Iowa State Press; 2001:50-59.