Signalment:
Histopathologic Description:
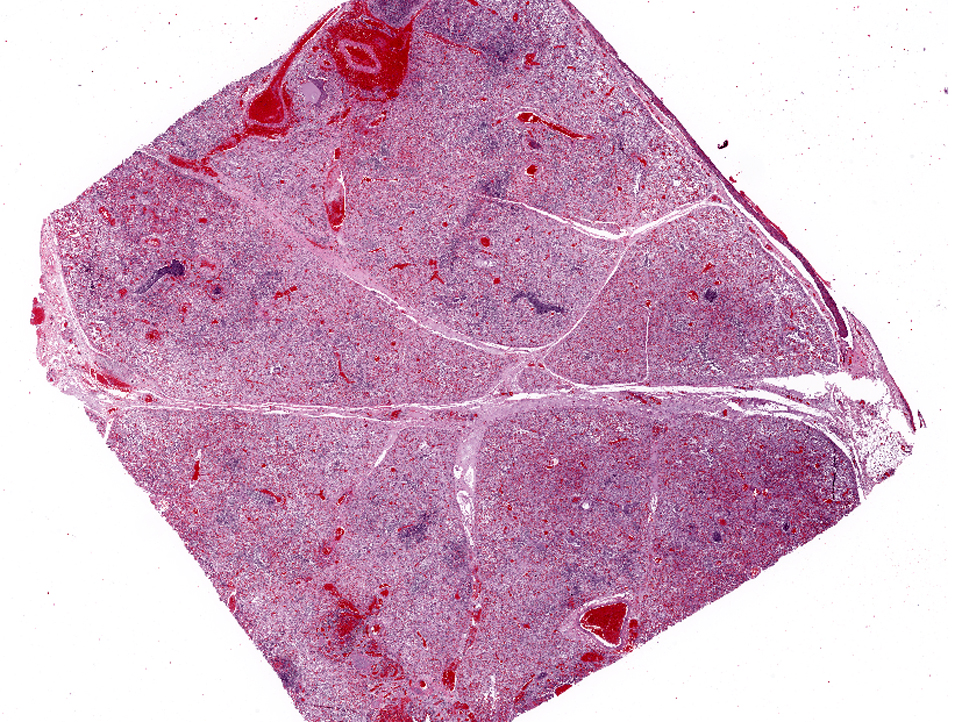
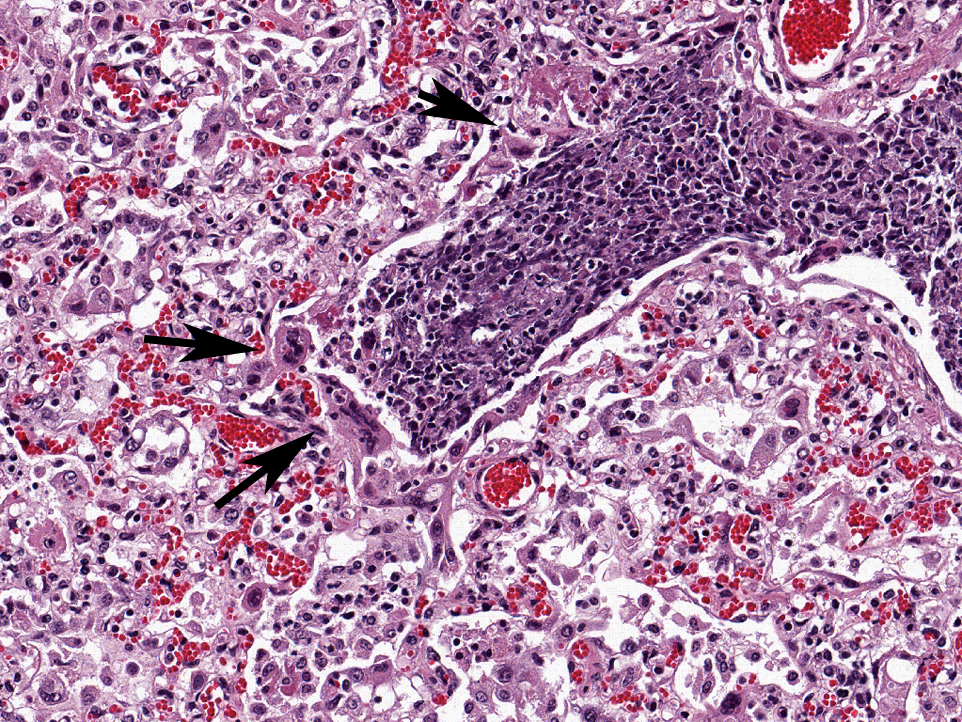
Affecting the pulmonary parenchyma is multifocal to coalescing, fibrinous and necrotizing bronchopneumonia. Alveoli contain variable amounts of viable and degenerate neutrophils, round "oat cells" with streaming nuclear contents, mats of fibrin, eosinophilic proteinaceous edema fluid, and intact alveolar macrophages. Occasional colonies of small coccobacilli are admixed with necrotic debris within alveoli. Alveolar septa are markedly expanded by fibrin, edema, and neutrophils. There are rare fibrin thrombi. The pleura and interlobular septa are markedly expanded by fibrin, edema, and suppurative infiltrates. Mats of fibrin, degenerate cellular debris, and viable and degenerate neutrophils line the pleural surface.
Morphologic Diagnosis:
Lung: 1) Marked, multifocal to coalescing, fibrinosuppurative bronchopneumonia.Â
Lung: 2) Marked, multifocal, necrotizing bronchiolitis with intralesional syncytia.
Lab Results:
Condition:
Contributor Comment:
The classic presentation of BRD in beef animals is pneumonia affecting multiple calves three days to three weeks after shipment from the farm of origin to the feedlot.(5) Risk factors in this group include: mixing of na+�-�ve calves from various sources; stress due to weaning, transport, crowding, handling, vaccination, dehorning, and food/water deprivation; metabolic acidosis due to diet change; and exposure to adverse or rapidly changing environmental conditions. In one-to-four-month-old dairy and veal calves, cases may be sporadic, enzootic, or epizootic.(5) In this group, risk factors include: poor air quality due to indoor housing, crowding, poor ventilation, and high humidity; reduced defenses due to failure of passive transfer of immunoglobulin; exposure to viral or other pathogens from co-housing with adults; and stress due to concurrent diseases or weaning.(5,6) Clinical signs include depression, pyrexia, tachypnea, dyspnea, and anorexia. While animals may respond to early antimicrobial therapy, relapse is common.(5)
This case includes lung from one of two submitted eight-month-old Holstein calves that demonstrated open mouth breathing and acute onset of respiratory disease. The histologic features are consistent with a viral and bacterial etiology. Differentials for the bronchiolar epithelial necrosis and epithelial syncytia formation included BRSV and PI-3. Polymerase chain reaction detected BRSV nucleoprotein. The severe fibrinosuppurative bronchopneumonia, coagulative necrosis, and leukocyte necrosis ("oat cells") is suspicious for Mannheimia hemolytic or Histophilus somni. Many colonies of M. hemolytic were obtained via general bacterial cultures of lung.
Bovine respiratory syncytial virus (BRSV) is of the genus Pneumovirus in the family Paramyxoviridae. It is a major cause of respiratory disease and a major contributor to BRD.(1) The infection is common in North America and Europe, within both beef and dairy operations. The seroprevalence of BRSV in adult dairy and beef cattle ranges from 40-95%.1,2 BRSV is an important cause of respiratory disease in two-week to five-month-old beef and dairy calves, and epidemics involving adult cattle have been reported.(3)
The severity of disease caused by BRSV depends on the age and immune status of the calf, route of infection, and strain of the virus.(3) Aerosol spread is the most common route of infection. The virus replicates in the epithelial cells of the upper respiratory tract, including the nasal cavity, pharynx, trachea, bronchi and bronchioli, and type II pneumocytes and alveolar macrophages. Significant effects include loss of cilia, necrosis of bronchial and bronchiolar epithelial cells, and depressed opsonization and phagocytosis by alveolar macrophages.(3) While specific serum and mucosal antibody responses occur in response to BRSV, the antibody is nonneutralizing and serum titers are poorly correlated with protection from disease.(2) A biphasic clinical course that is observed in some natural infections may represent a worsening of the disease following an immune response; however, the enhancement of the disease process by the immune system is controversial.(2) Viral shedding following experimental infection of na+�-�ve calves occurs between two and eight days post-infection. Viral antigen is most abundant in the cranioventral lung.Â
Most BRSV infections are subclinical. Mildly to severely affected dairy calves may demonstrate fever, cough, nasal/oral/ocular discharge, and tachypnea.(3) Severely affected animals may exhibit severe respiratory distress, including dyspnea, forced grunting upon expiration, and increased abdominal effort.Â
Gross findings of BRSV infections differ between cranial and caudal portions of lung. The cranioventral lung is grossly deep red to mottled, atelectatic, collapsed, and rubbery in texture.(1,2) The caudodorsal lung may be heavy, edematous, or more firm than normal, with or without emphysema.(1,2) These gross lesions are widely variable, and may vary with concurrent bacterial bronchopneumonia. Lesions in other organs are not usually observed.(2)
Histologically, BRSV is characterized by necrotizing bronchiolitis, formation of bronchiolar epithelial syncytia, and exudative or proliferative alveolitis; these lesions are due to the cytopathic effects of the virus on epithelium, as previously described. Bronchioles are lined by attenuated epithelium, and multinucleated syncytial cells are frequently closely associated with these areas or found free in the lumina. Occasionally, alveoli may contain syncytia.(2) Bronchioles contain necrotic epithelial cells and neutrophils, and alveoli contain neutrophils and macrophages. Hyaline membranes may occur but are infrequent.(1,2) Intracytoplasmic eosinophilic inclusion bodies are occasionally present in syncytial cells.(2)
Differential diagnoses include bovine parainfluenza virus-3 (PI3) infection. In both diseases, syncytia with intracytoplasmic inclusion bodies occur. Generally, PI3 is associated with fewer syncytia located within the alveoli. Ancillary diagnostics are usually necessary for definitive diagnosis of BRSV, due to the confounding nature of multiple pathogens in the bovine respiratory disease complex. Diagnosis may be confirmed using fluorescent antibody tests, immunohistochemistry, antigen-detecting ELISA, or real time reverse transcriptase PCR(qRT-PCR).(1,2) qRT-PCR is considered the gold standard, based on its speed and sensitivity.(1)
Mannheimia hemolytic is a member of the Pasteurellaceae family. M. hemolytic is a small gram-negative coccobacillus that exists in the upper respiratory tract of healthy ruminants, primarily within the nasopharynx and tonsillar crypts.(4,5) Twelve serotypes exist, and serotypes A1 and A5 are known to colonize the upper respiratory tract of cattle and sheep as part of the normal commensal flora.(4) Serotype A1 quickly becomes the predominant organism once the commensal relationship is disrupted, and this serotype is also frequently isolated from pneumonic tissue.(4,5) Stress, exposure to cold, and concurrent viral illness and decreases in lung defenses are possible causes for the shift from a commensal relationship to pathologic one. The host response to the infection is the primary cause of clinical disease.(5) Virulence factors include leukotoxin, lipopolysaccharide, capsular polysaccharide, transferring-binding proteins A and B, O-sialogylcoprotease, neuraminidase, IgG1-specific protease, outer membrane proteins, and fimbriae.(5) These virulence factors allow M. hemolytic to evade clearance, avoid host defenses, and impair leukocyte function.(4,5) The result is massive recruitment of neutrophils into alveoli that are ineffective at killing bacteria but cause significant endothelial damage to capillaries and small vessels resulting in vascular leakage of protein, fluid, and fibrin into alveolar and interstitial spaces.(4,5,8)
Gross lesions caused by M. hemolytic include cranioventral lobar or lobular fibrinous bronchopneumonia, foci of coagulative necrosis, and variable fibrinous pleuritis.(5) Variable amounts of straw-colored thoracic fluid may be present.(4) On cut surface, the lung may be described as "meaty," being dark purple to red, heavy, firm or hard, and moist.(4,5)
Histopathologic findings of M. hemolytic pneumonia include fibrinous and suppurative bronchopneumonia with necrosis of leukocytes.(5) Necrotic leukocytes, including neutrophils and macrophages, are lytic, and exhibit a streaming pattern of pale basophilic chromatin; as this pattern resembles oats, the term "oat cells" is commonly used.(5) Alveoli and airways contain variable amounts of viable and degenerate neutrophils and macrophages, fibrin, edema, erythrocytes, colonies of bacteria, and necrotic debris. Fibrin thrombi are frequently present, and interlobular septa are distended by fibrin, edema, and inflammation. Sharply demarcated regions of coagulative necrosis are often present.(5) Differential diagnoses for the gross and histopathologic lesions include H. somni and P. multocida. Evidence of bronchiolar necrosis is suggestive of an underlying viral infection; however, neutrophil-mediated damage may also cause such lesions.(5)
In the diagnostic setting, definitive diagnosis is determined by aerobic bacterial culture. However, prior treatment of the animal with antimicrobials frequently results in failure to culture bacterial agents. As well, opportunistic and secondary bacteria, including Arcanobacterium progenies and Mycoplasma species, may be present and confound the results.
JPC Diagnosis:
1. Lung: Bronchopneumonia, necrotizing, diffuse, moderate, with epithelial viral syncytia and intracytoplasmic viral inclusion bodies.
2. Lung: Bronchopneumonia, fibrinosuppurative, diffuse, moderate, with oat cells, numerous bacteria, and fibrinosuppurative pleuritis.
Conference Comment:
The contributor mentioned leukotoxin, a primary virulence factor produced by M. hemolytic, which is a repeats in toxin (RTX) exotoxin. RTX is named for the presence of a tandemly-repeated nine-amino acid residue sequence in the protein, specifically glycine-rich nonapeptides, which bind Ca2+ on the C-terminal half of the protein. RTX toxins are pore-forming protein toxins produced by a wide range of pathogenic gram-negative bacteria that result in necrosis or apoptosis and are generally classified as hemolytic, cytotoxic or both. Other important RTX toxins in veterinary medicine include hemolysin produced by Escherichia coli, ApxI and ApxII produced by Actinobacillus pleuropneumoniae, leukotoxin produced by Pasteurella multocida, and adenylate cyclase toxin produced by Bordetella bronchiseptica.(2,5)
References:
2. Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Philadelphia, PA: Saunders Elsevier; 2007:596 598.Â
3. Vuuren MV. Bovine respiratory syncytial virus infection. In: Coetzer JAW, Tustin RC, eds. Infectious Diseases of Livestock. 2nd ed. Toronto, CA: Oxford University Press, 2004:677 679.
4. Griffin D, Chengappa MM, Kuszak J, et al. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet Clin North Am Food Anim Pract. 2010;26(2):381 394.Â
5. Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Philadelphia: Saunders Elsevier; 2007:587-8, 601604.Â
6. Gorden PJ, Plummer J. Control, Management, and Prevention of Bovine Respiratory Disease in Dairy Calves and Cows. Vet Clin North Am Food Anim Pract. 2010;26(2):243259.Â
7. Griffin D. Bovine Pasteurellosis and Other Bacterial Infections of the Respiratory Tract. Vet Clin North Am Food Anim Pract 2010;26(2):5771.Â
8. Ackermann MR, Brogden KA. Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica. Microbes Infect. 2000;2:10791088.Â