Signalment:
Juvenile, female, spayed ferret (
Mustela putorius furo).Approximately six months after arrival from a commercial vendor, this ferret became progressively anorexic with weight loss, diarrhea, and had palpable intra-abdominal masses. There was poor response to IBD-type treatments and the ferret was euthanized.
Gross Description:
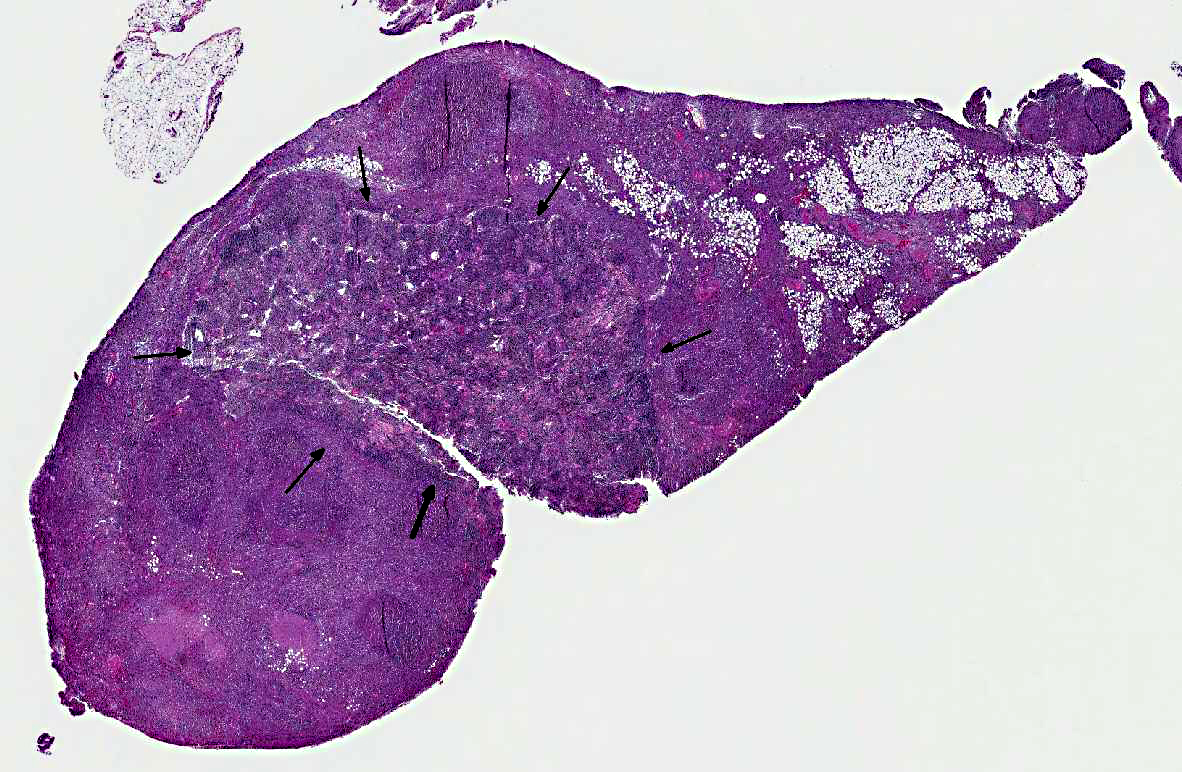
An approximately 3 x 3 x 5 cm multi-lobular white-tan mass was present in the area of the mesenteric lymph nodes and surrounding mesenteric fat.Â
Histopathologic Description:
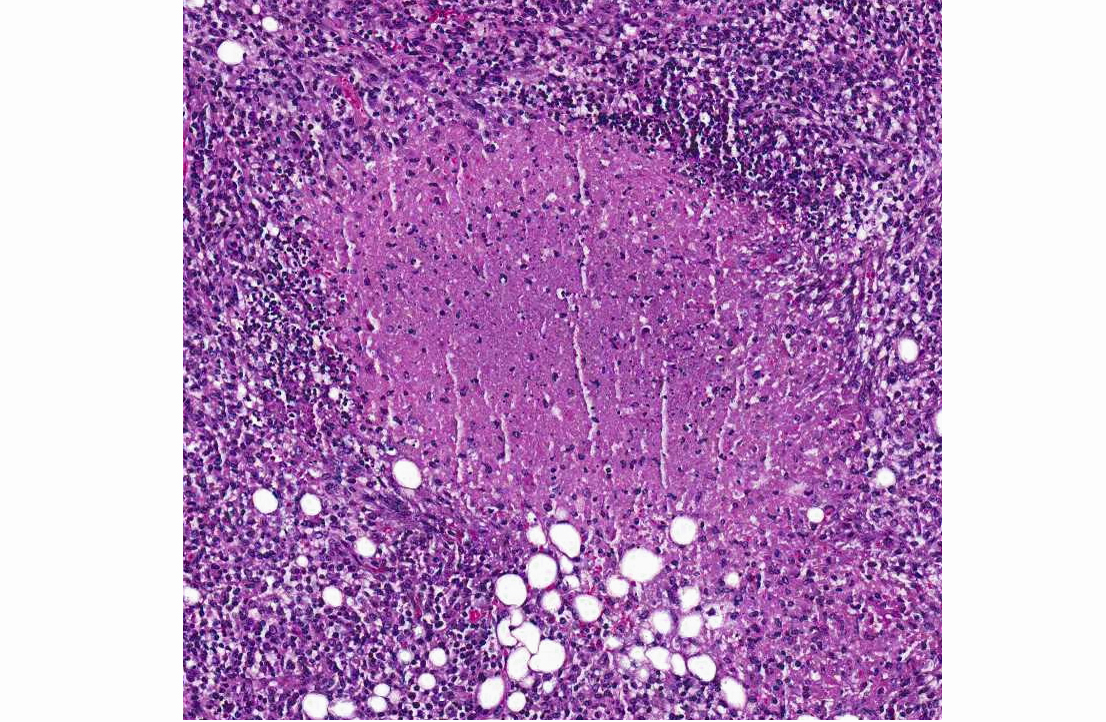
Mesenteric lymph node and mesentery: The lymph node and mesenteric fat are effaced by multifocal to coalescing pyogranulomatous infiltrates. There are large central regions of necrosis with admixed degenerate neutrophils and fibrin surrounded concentrically by epithelioid macrophages and fibroblasts with fewer peripheral lymphocytes, plasma cells, and rare multinucleated giant cells. Several large arteries are partially or completely occluded by fibrin thrombi and portions of the wall are replaced by inflammatory cells and debris.Â
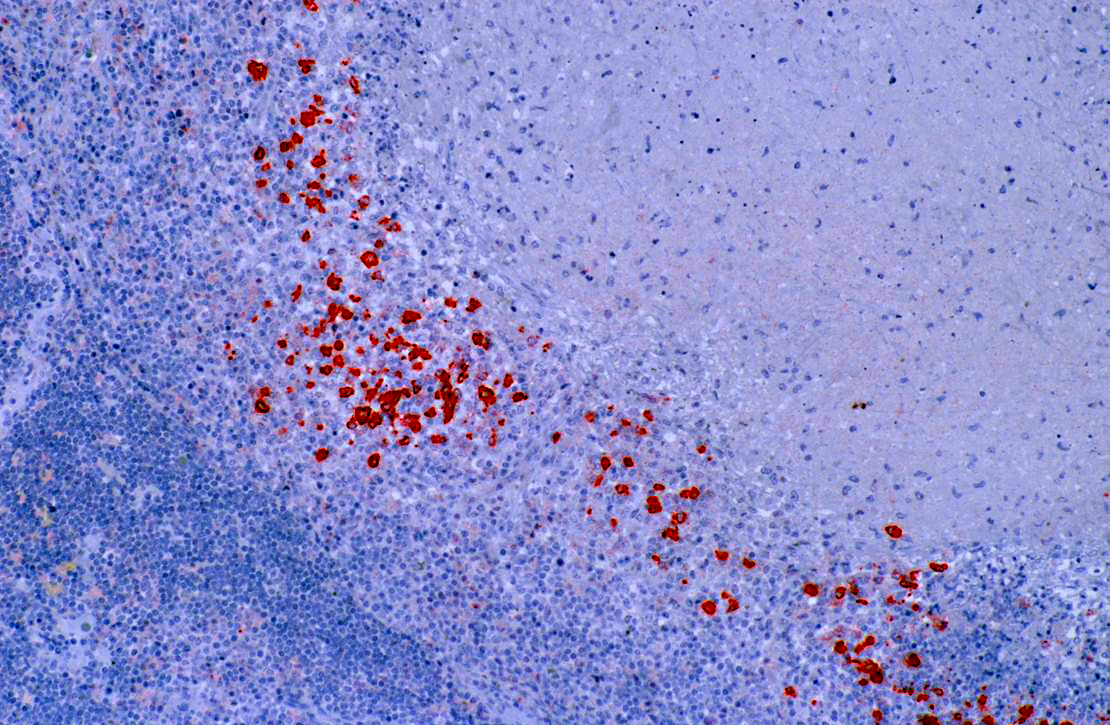
Coronavirus immunohistochemistry performed at Michigan State University using a monoclonal antibody against group 1c coronavirus antigen reveals strong positive intracytoplasmic staining of macrophages within the center of pyogranulomas.
Morphologic Diagnosis:
Mesenteric lymph node and mesenteric fat: Severe multifocal to coalescing pyogranulomatous and necrotizing lymphadenitis, peritonitis, and vasculitis.Â
Condition:
Coronavirus-associated granulomatous disease
Contributor Comment:
The primary differential etiology for this animal from gross and microscopic lesions was ferret systemic coronavirus infection which was subsequently confirmed by immunohistochemistry. Both the ferret enteric coronavirus (FRECV) and the ferret systemic coronavirus (FRSCV) were recently identified as group 1 coronaviruses.(3,4) Ferret enteric coronavirus causes an enteric disease called epizootic catarrhal enteritis (ECE).(4) More recently, a new systemic coronavirus-associated disease closely resembling the granulomatous or dry form of feline infectious peritonitis (FIP) has been reported.(1) Although the similarities in clinical disease and histologic lesions between FRSCV and FIP virus suggest a similar pathogenesis for FRSCV-associated disease and FIP, this has yet to be proven experimentally.Â
JPC Diagnosis:
Lymph node: Lymphadenitis, necrotizing and pyogranulomatous, multifocal to coalescing, severe, with mild fibrinoid vasculitis and necrotizing mesenteric steatitis.Â
Conference Comment:
Viruses in the family
Coronaviridae, genus
Coronavirus, are 80-220 nm, enveloped and often spherical (although they can be pleomorphic), with large club-shaped viral spike peplomers (S protein) surrounding an icosahedral core that contains a helical nucleocapsid (N protein). These viruses consist of a single molecule of positive single-stranded RNA.(2) Coronavirus infections have been reported in many species, including pigs, cattle, horses, cats, frogs, rats, birds, rabbits, ferrets, mink, and mice. The host spectrum of each coronavirus depends on the S protein, which mediates receptor binding and fusion of the virus with the host cell. Coronaviruses use different receptors, including aminopeptidase N, used by several group 1 coronaviruses; carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1), used by mouse hepatitis virus; and N-acetyl-9-O-acetyl neuraminic acid, used by other group 2 coronaviruses. Group 3 coronavirus receptors are unknown at this time; however, heparan sulfate and sialic acid residues may play a role as non-specific attachment factors.(2) Coronviruses of veterinary importance include:
Group 1a:
Transmissible gastroenteritis virus of swine
Porcine respiratory coronavirus
Canine coronavirus
Feline enteric coronavirus (formerly feline infectious peritonitis virus)
Ferret and mink coronaviruses
Group 1b:
Porcine epidemic diarrhea virus
Bat coronavirus
Group 2a:
Mouse hepatitis virus
Bovine coronavirus
Sialodacryoadenitis virus of rats
Porcine hemagglutinating encephalomyelitis virus
Canine respiratory coronavirus
Group 3:
Avian infectious bronchitis virus
Turkey coronavirus(2)
In carnivores infected by coronaviruses, disease manifests in one of two ways: infection can be self-limiting enteritis (such as in canine coronavirus, feline coronaviral enteritis, ferret epizootic catarrhal enteritis), or severe systemic disease can occur (such as in feline infectious peritonitis [FIP] or ferret systemic coronavirus infection [FRSCV].)(1) As the contributor noted, pathogenesis for FRSCV is suspected to be similar to that of FIP, based on gross, histologic and immunohistochemical features of the disease.(1) The key feature in FIP is the ability of genetic variants of feline enteric coronavirus to infect macrophages, due to mutations of the S protein and possibly other proteins which alters the tropism of the virus. Strong antibody response (often evidenced clinically as a polyclonal gammopathy) is ineffective at eliminating the virus and cellular immune responses are unable to prevent virus replication in macrophages. The lesions in FIP are often centered on small blood vessels, with vascular injury (necrosis) and leakage of a viscous, protein-rich transudate playing a major role in the wet form of the disease.(2) In this case of ferret systemic coronavirus infection, conference participants noted that, although there was significant slide variation, in some specimens, variable amounts of necrotizing vasculitis are visible. Despite this finding, ferrets generally do not develop the effusive (wet) form of the disease, as they more often present with lesions consistent with the non-effusive (dry) end of the disease spectrum.(1)
References:
1. Garner MM, Ramsell K, Morera N, et al. Clinicopathologic features of a systemic coronavirus-associated disease resembling feline infectious peritonitis in the domestic ferret (
Mustela putorius).Â
Vet Pathol. 2008;45:23646.
2. Maclachlan NJ, Dubovi EJ, eds. Coronaviridae. In:
Fenner's Veterinary Virology. London UK: Elsevier Science; 2010:394-413.
3. Wise A, Kiupel M, Garner MM, et al. Comparative sequence analysis of the distal one-third of the genomes of a systemic and an enteric ferret coronavirus.Â
Virus Res. 2010;149:4250.
4. Wise AG, Kiupel M, Maes RK. Molecular characterization of a novel coronavirus associated with epizootic catarrhal enteritis (ECE) in ferrets.Â
Virology. 2006;349:16474.