Signalment:
31-year-old, female, rhesus macaque (
Macaca mulatta).This rhesus macaque developed vaginal bleeding 13 months prior to euthanasia. Pap smear revealed
atypical epithelial cells with large, irregularly shaped, vesicular nuclei. Colposcopic examination was suggestive of
cervical intraepithelial neoplasia at the cervico-vaginal junction. Surgical removal of the cervix was performed 1.5
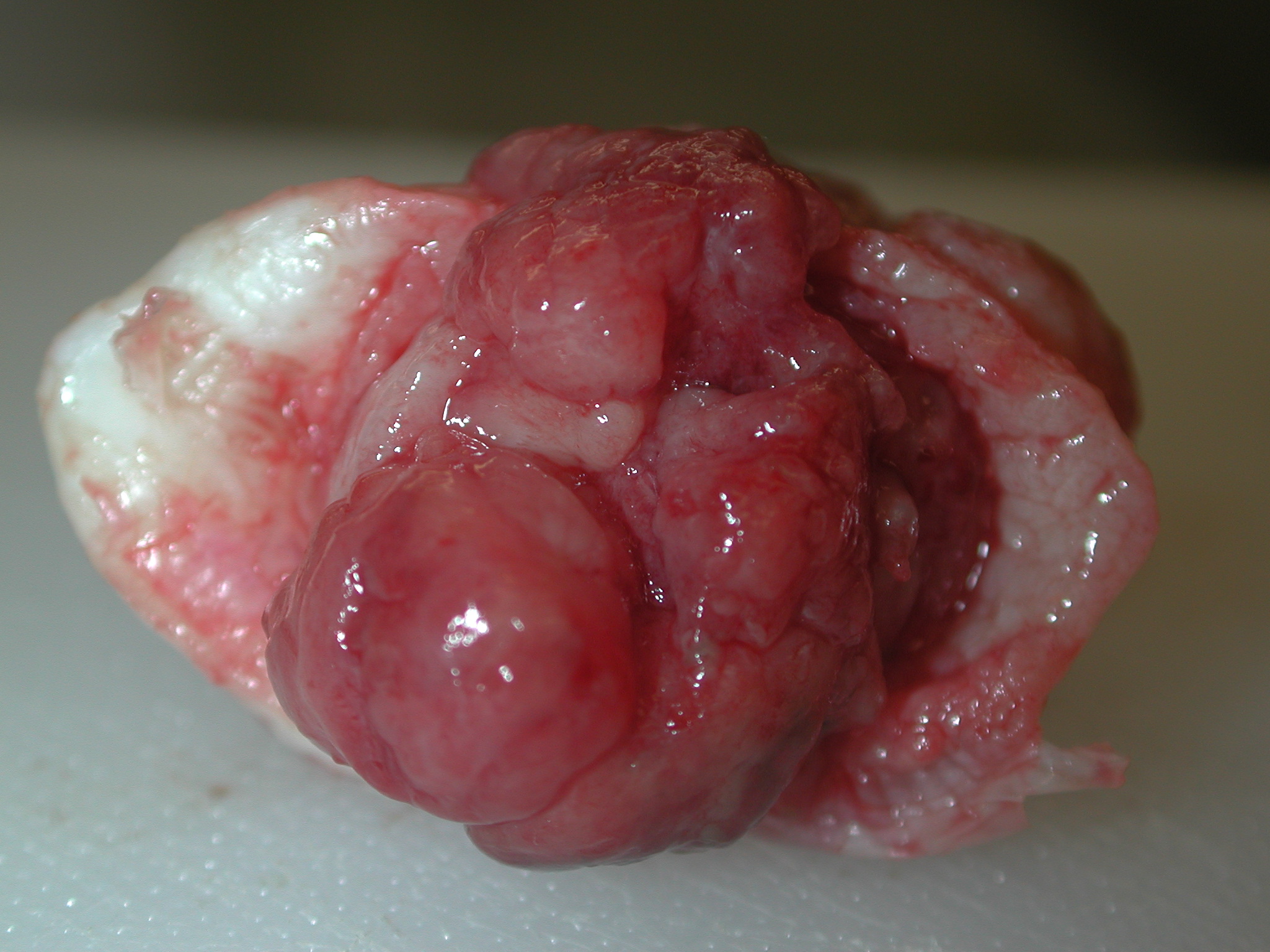
months later, and a 2.0 x 1.5 x 0.6 cm, multilobular, unencapsulated, bright red, fleshy, mass was found at the
external cervical os near the cervico-vaginal junction. Histological examination was consistent with cervical
adenocarcinoma. Nearly a year later the animal became anorexic and constipated, and was euthanized.
Gross Description:
At necropsy the distal colon was constricted and adhered to the urinary bladder, and a 7 x 5 x 5
mm, unencapsulated, reddish-brown mass infiltrated the vaginal mucosa adjacent to the cervical stump. Multifocal
to coalescing, tan to milky white plaques were present on the serosa of the urinary bladder and colon. Gross
morphologic diagnoses: Cervical adenocarcinoma with serosal metastasis to the colon and urinary bladder.
Histopathologic Description:
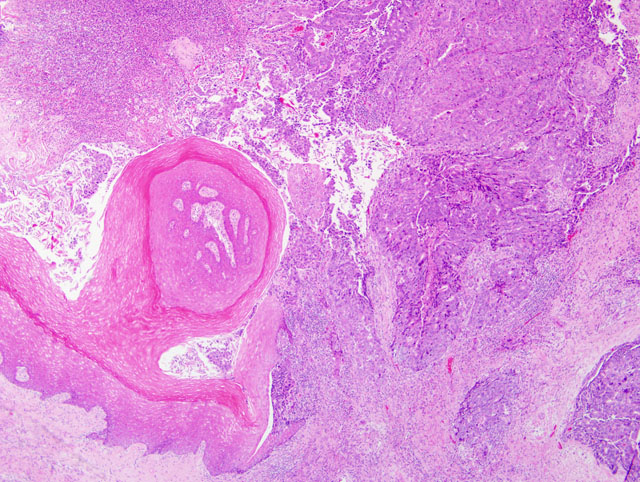
Extensively infiltrating and replacing the vaginal wall is part of an unencapsulated,
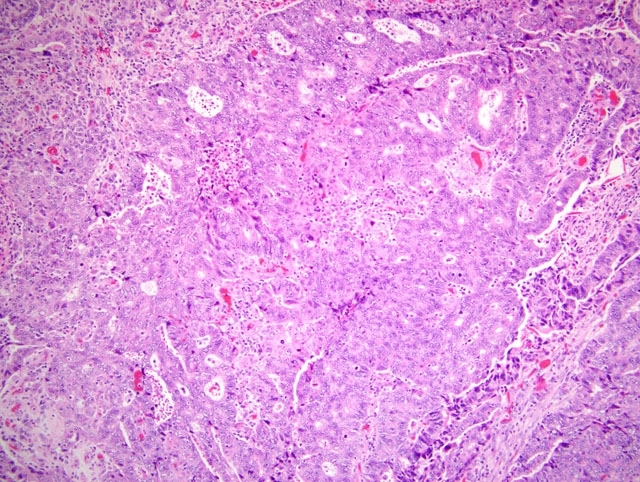
poorly demarcated, densely cellular epithelial neoplasm. The neoplastic cells are arranged in solid sheets, acini, and
nests supported by a fine fibrovascular stroma. The cells are often irregularly multilayered, range from oval to
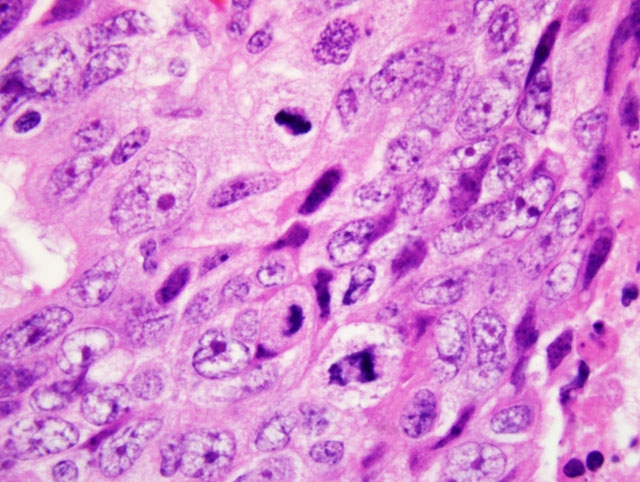
polygonal or columnar, have indistinct cell borders, moderate to abundant pale eosinophilic, homogenous or finely
vacuolated cytoplasm, and vesicular, oval nuclei with 1-2 basophilic nucleoli. Anisokaryosis and anisocytosis are
moderate to marked, and mitoses range from 3-8 per 40x HPF. Invasion of the lymphatics, colonic serosa,
muscularis and submucosa is present multifocally, although it is not present in all sections. The surface of the
neoplasm is variably covered by a 2 mm thick sheet of fibrin and necrotic debris admixed with degenerate
neutrophils and small basophilic bacterial colonies.
Morphologic Diagnosis:
Cervical adenocarcinoma with vaginal and colonic invasion.
Lab Results:
A PCR on Pap smear tissue using a rhesus papillomavirus D sequence was positive.
Condition:
Cervical adenocarcinoma (rhesus papillomavirus D)
Contributor Comment:
Papillomaviruses are a diverse group of epitheliotropic double-stranded DNA viruses, of
which more than 75 have been identified in 20 species. The rhesus papillomavirus type D (RhPV-d) is the most
common isolate associated with genital infections in macaques, and was associated with 60% of genital lesions
diagnosed in rhesus macaques,(7) which include vaginal papillomas, varying stages of intraepithelial dysplasia, and
invasive cervical carcinoma.(6) Infection requires the availability of epidermal or mucosal epithelial cells still able
to proliferate (basal cells).(9) Histological characteristics include koilocytosis, epithelial atypia and loss of basal cell
maturation.
Cervical adenocarcinoma associated with genital papillomaviruses has been well described in human medical
literature and recently in macaques.(6-8) The normal cervix has two distinct epithelial zones, an ectocervix covered
by squamous epithelium, and an endocervix lined by simple glandular epithelium. During adolescence, the
endocervical epithelium undergoes squamous metaplasia and is replaced by immature squamous epithelial cells
which later undergo maturation. This metaplastic region is called the transformation zone and is the most common
site for the development of cervical cancer.(3) High grade lesions often occur at the squamo-columnar junction. In
humans, nearly 80 percent of the population is infected by genital papillomaviruses, which are considered the most
prevalent sexually transmitted ongocenic pathogens. Only rarely does infection lead to invasive cervical carcinoma
which is characterized as squamous cell carcinoma (SCC) or adenocarcinoma (AC). Human papillomavirus (HPV)
16 is the most frequent isolate in SCC, while HPV 18 is detected more frequently in AC. In many human cases, the
cervical lesions harbor multiple HPV types.(8)
Human papillomavirus integration into the genome leads to inactivation of the p53 and retinoblastoma (Rb)
pathways by the action of primary papillomaviral oncoproteins E6 and E7. Viral E6 protein binds to and degrades
p53, and viral E7 protein causes functional inactivation of the Rb protein. High-risk HPV infections are associated
with increased expression of E6 and E7 genes in precancerous lesions.(2) E5 is another gene important in the early
course of infection as it stimulates cell growth by binding the epidermal growth factor receptor, the platelet-derived
growth factor-β receptor, and the colony-stimulating factor-1 receptor. Recently, E5 has also been shown to prevent
apoptosis following DNA damage.(9)
JPC Diagnosis:
Cervicovaginal junction: Cervical adenocarcinoma
Conference Comment:
As one of very few suitable animal models for one of the leading causes of cancer
mortality in women worldwide, this entity is extremely relevant and timely. Furthermore, the contributor provides a
superb synopsis of its pathogenesis. Readers may recall a recent discussion of the role of oncoproteins E5, E6, and
E7 in neoplastic transformation in bovine enzootic hematuria associated with bovine papillomaviruses 2 and 4 and
bracken fern (see
WSC 2009-2010, Conference 10, case IV). Not surprisingly, oncoproteins E5 and E7 are
expressed in and have been implicated in the pathogenesis of equine sarcoid, a condition of which bovine
papillomaviruses 1 and 2 are the suspected etiologies. The E5 oncoprotein binds the platelet-derived growth factor
beta receptor, which is also expressed in equine sarcoids.(1) In general, papillomaviruses characteristically exhibit
marked species specificity, and equine sarcoid is one of the rare exceptions to this tendency. Recently, feline
sarcoid-associated papillomavirus DNA sequences have been amplified from bovine skin, suggesting that cattle may
be the reservoir host of this papillomavirus.(4) Finally, RhPV-d and RhPV-a, previously found only in rhesus
macaques, recently were isolated from the genital tracts of female cynomolgus macaques.(6,7)
References:
1. Borzacchiello G, Russo V, Della Salda L, Roperto S, Roperto F: Expression of platelet-derived growth factorbeta
receptor and bovine papillomavirus E5 and E7 oncoproteins in equine sarcoid. J Comp Pathol
139:231-237,
2008
2. Dray M, Russell P, Dalrymple C, Wallman N, Angus G, Leong A, Carter J, Cheerala B: P16(INK4a) as a
complementary marker of high-grade intraepithelial lesions of the uterine cervix. I: Experience with squamous
lesions in 189 consecutive cervical biopsies. Pathology
37:112-124, 2005
3. Jenkins D: Histopathology and cytopathology of cervical cancer. Dis Markers
23:199-212, 2007
4. Munday JS, Knight CG: Amplification of feline sarcoid-associated papillomavirus DNA sequences from bovine
skin. Vet Dermatol [Epub ahead of print], 2010 Mar 29
5. Ogura K, Ishi K, Matsumoto T, Kina K, Nojima M, Suda K: Human papillomavirus localization in cervical
adenocarcinoma and adenosquamous carcinoma using in situ polymerase chain reaction: Review of the
literature of human papillomavirus detection in these carcinomas. Pathol Int
56:301-308, 2006
6. Wood CE, Borgerink H, Register TC, Scott L, Cline JM: Cervical and vaginal epithelial neoplasms in
cynomolgus monkeys. Vet Pathol
41:108-115, 2004
7. Wood CE, Chen ZG, Cline JM, Miller BE, Burk RD: Characterization and experimental transmission of an
oncogenic papillomavirus in female macaques. J Virol
81:6339-6345, 2007
8. Zuna RE, Allen RA, Moore WE, Mattu R, Dunn ST: Comparison of human papillomavirus genotypes in highgrade
squamous intraepithelial lesions and invasive cervical carcinoma: evidence for differences in biologic
potential of precursor lesions. Mod Pathol
17:1314-1322, 2004
9. zur Hausen H: Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer
2:342-350, 2002