Signalment:
S370/10: Adult male budgerigar (Melopsittacus undulatus).In August 2010, sudden deaths of parakeets were noticed in an aviary close to Berlin, Germany. Three barred parakeets (Bol-borhynchus lineola) and two budgerigars (Melopsittacus undulatus) died within 2-5 days after a clinical history of reduced activity and food intake. Additionally, one barred parakeet and two juvenile budgerigars were clinically affected for about two weeks but finally recovered. During the past two years, new parakeets had not been introduced into the aviary.
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Genetic analysis suggests Haemoproteus spp. from European songbirds as causative agents in both cases presented here.9 In Europe, asymptomatic blood infections by Haemoproteus spp. have been regularly observed and are especially prevalent in songbirds.12 In the German outbreak, psittacine species endemic to South Africa and Australia were infected with two different lineages of Haemoproteus spp. that are known to infect blackbirds and songthrushes (Turdidae), respectively. These results suggest that infection was the result of previously unknown cross-species transmission of Haemoproteus spp. between birds of only distantly related phylogenetic orders.7,11 Haemoproteus spp. are closely related to Plasmodium spp. causing avian malaria.1 Whether the term avian malaria should be used for haemosporidian parasites other than Plasmodium spp. is still subject of discussion.8,14 We propose to term the disease in parrots Haemoproteosis (G. Valkiunas, personal communication).14
The Haemoproteus spp. identified are highly prevalent in the native European songbird population but normally do not cause harm to their hosts. In contrast, the cases reported here suggest that the same parasites may cause overt disease in invading species such as exotic parrots. These parasites that have adapted to European songbirds may cause fatal outbreaks in native psittacines of Australia, New Zealand, and South America that are raised in captivity. Blood-sucking insects such as biting midges (Culicoides), the vectors for Haemoproteus spp. of passerine birds in Europe may transmit the parasite from songbirds to parrots.8 Since no gametocytes in parrot blood have been found and no blood stages have ever been described in previous reports, a completely abortive development has to be assumed. The pathogenesis in parrots is unknown. Presumably, tissue damage caused by megalomeronts in the conduction system of the heart may cause death in the affected birds.
Morphologically, the parasitic structures reported here were strikingly similar to the reported cases of numerous previous outbreaks in Europe. However, further retrospective, epidemiologic and experimental studies are needed to assess the full range of Haemoproteus spp. or other hematozoan parasites involved in this disease of parrots. Since blackbirds and songthrushes have been introduced to Australia and New Zealand in the 19th century, there is a concern that these parasites already have established in these areas and are potentially affecting the natural population of parrots.
JPC Diagnosis:
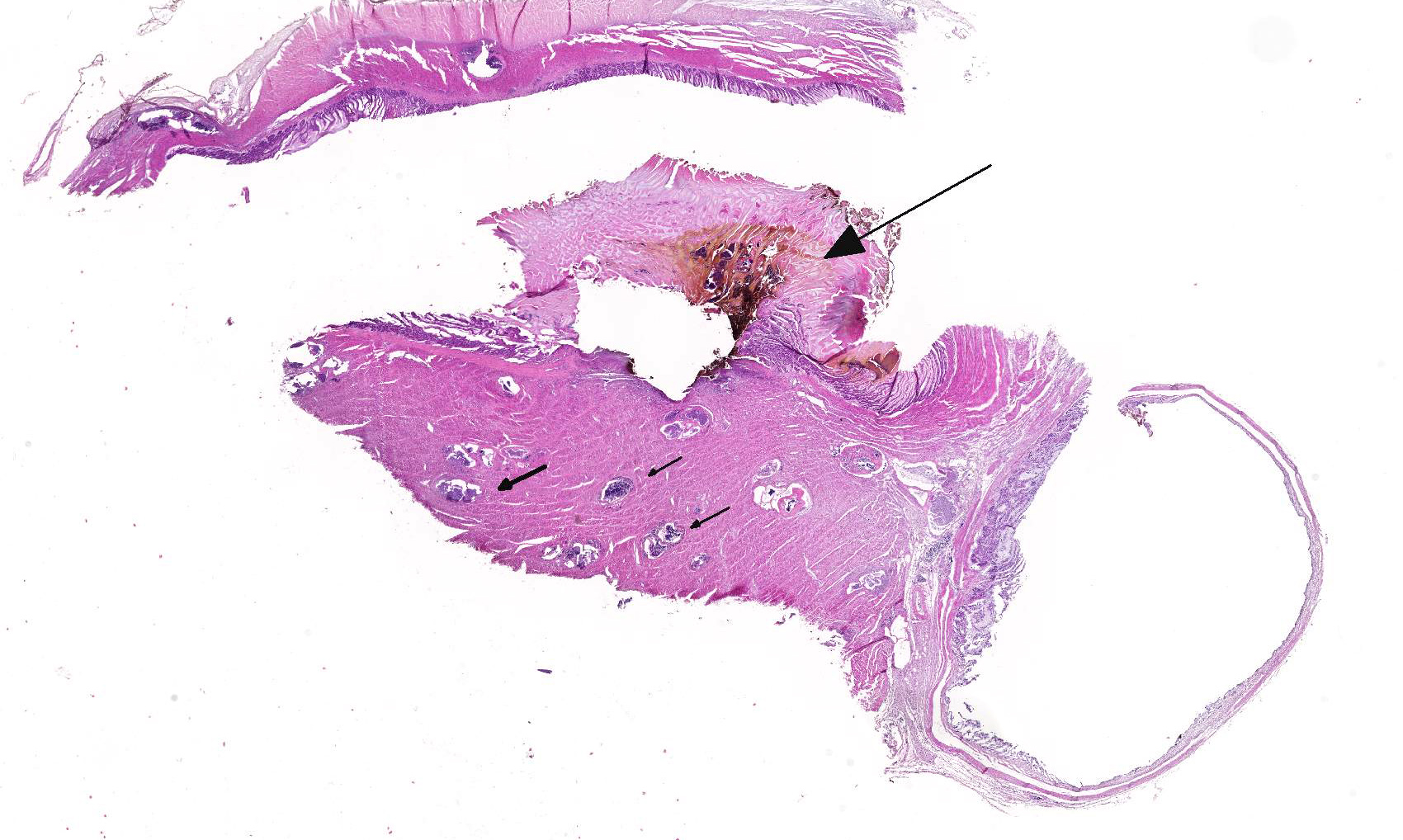
2. Proventriculus: Proventriculitis, ulcerative, focal, moderate with hemorrhage.
Conference Comment:
Within the avian host, the sporozoites enter the vascular endothelial cells, most often within the lung, liver, bone marrow, and spleen where they undergo schizogony to form clusters of schizonts filled with merozoites. Sexual stages occur within the arthropod vector, while asexual reproduction occurs within the vertebrate host. Once the schizont ruptures, merozoites are released into the circulation, where they infect host red blood cells. Within erythrocytes, merozoites transform into macro or microgamonts. Hemoproteus infection is often subclinical in birds and clinical disease is associated with anemia due to parasitism of the host red blood cells. Additionally, clinically affected animals are usually concurrently immunocompromised. Severely affected animals can acutely die with no overt clinical signs.3,5,10
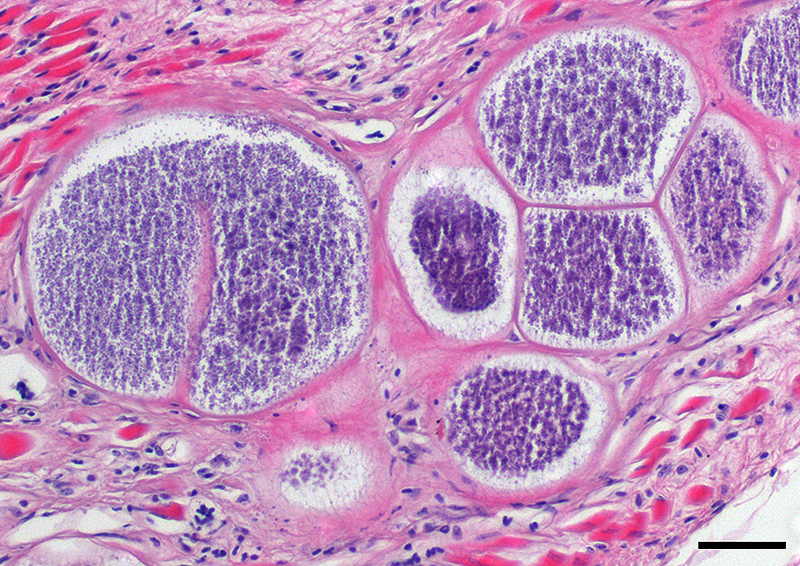
This case likely represents a manifestation of Haemoproteus infection associated with the pre-erythrocyte stage, characterized by large megaloschizonts within both the skeletal and smooth muscle. The conference moderator noted that in this stage, intra-erythrocytic gametocytes are not seen, consistent with the history provided by the contributor. Rather than anemia secondary to erythrocyte parasitism, lesions are associated with megaloschizonts within a variety of cell types, such as the muscular layers of the proventriculus and skeletal muscle, in this case.3,5 The conference moderator also noted that similar to previously reported cases, the mega-loschizonts in this case are large (200-500 um), have compartmentalized internal septae, and are often associated with hemorrhage.5 Within mature mega-loschizonts, there are numerous packeted merozoites within structures known as cytomeres. As they mature, megaloschizonts rupture and merozoites are released into the blood stream. These merozoites then infect erythrocytes and become gametocytes, ready to be ingested by biting flies to complete the life cycle of the parasite.3,5,10 In previously reported cases, rupture of megaloschizonts and the release of merozites caused intense inflammation, hemorrhage, and necrosis5; however, in this case there is only very mild inflammation. It is unclear if the ulcerative proventriculitis with hemorrhage and hemosiderin laden macrophages in the koilin membrane is related to the parasite. Despite the relatively mild lesions present in these tissue sections, conference participants agreed with the contributor that there were likely megaloschizonts in the heart that interfered with signal conduction, ultimately leading to the death of these birds.
References:
1. Atkinson CT, Van Riper CI. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M eds. BirdParasite Interactions: Ecology, Evolution, and Behavior. New York: Oxford University Press; 1999:19-48.
2. Bennett GF, Peirce MA, Ashford RW. Avian haematozoa: Mortality and pathogenicity. J Nat Hist.1993; 27:993-993.
3. Donovan TA, Schrenzel M, Tucker TA, Pessier AP, Stalis IH. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: Eleven cases. J Vet Diagn Invest. 2008; 20:304-313.
4. Frank W, Kaiser L. Infection with parasites of the genus Leucocytozoon (Protozoa, Sporozoa) in the breeding of parrots (Aves, Psittaciformes). Zeitschrift für Parasitenkunde (Berlin, Germany) 1967; 28:370-382.
5. Gardiner CH, Fayer R, Dubey JP. Apicomplexa. In: An Atlas of Protozoan Parasites in Animal Tissues. 2nd ed. Armed Forces Institute of Pathology, American Registry of Pathology, Washington DC; 1998:73-75.
6. Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004; 90:797-802.
7. Krizanauskiene A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiunas G. Variation in host specificity between species of avian hemosporidian parasites: Evidence from parasite morphology and cytochrome B gene sequences. J Parasitol. 2006; 92:1319-1324.
8. Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol Phylogenet Evol. 2008; 47:261-273.
9. Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R. Avian malaria deaths in parrots, Europe. Emerg Infect Dis. 2011; 17:950-952.
10. Pennycott T, Wood A, MacIntyre C, MacIntyre D, Patterson T. Deaths in aviary birds associated with protozoal megaloschizonts. Vet Rec. 2006; 159:499-500.
11. Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. Proc Biol Sci. 2002; 269:885-892.
12. Scheuerlein A, Ricklefs RE. Prevalence of blood parasites in European passeriform birds. Proceedings of the Royal Society of Biological Sciences. 2004; 271:1363-1370.
13. Smith GA. Aberrant leucocytozoon infection in parakeets. Vet Rec. 1972; 91:106.
14. Valkiunas G, Anwar AM, Atkinson CT, Greiner EC, Paperna I, Peirce MA. What distinguishes malaria parasites from other pigmented haemosporidians? Trends Parasitol. 2005; 21:357-358.