Signalment:
17-year-old, neutered male, quarterhorse, (
Equus caballus).Horse developed severe atrophy of facial muscles on the left side
of the face 2 months prior to presentation to the teaching hospital. The weekend
prior to submission the patient developed rear limb ataxia. Probing palpation
of the cervical region revealed hyperesthesia and hyper-responsiveness. Probing
around the mid cervical region did not elicit a response. Treatment with
dexamethasone yielded some improvement in clinical signs. The horse was
euthanized at the owner elected request.
Gross Description:
The muscles on the left side of the face were diffusely atrophied
and hemorrhage was present in the anterior compartment of the right eye. There
was narrowing of the spinal canal between C2 and C3. The dura mater at the
C2-C3 articulation was focally reddened. The third cervical vertebral body (C3)
contained a 2.5 x 1.5 cm region of red and depressed tissue (bony
sequestration) rimmed by thick white tissue (fibrosis) at its ventral border.
The dorsal vertebral body of C2 also had a 1.5 cm linear band of firm white
tissue (fibrosis) that traversed the bone in a dorsal-ventral direction.
Histopathologic Description:
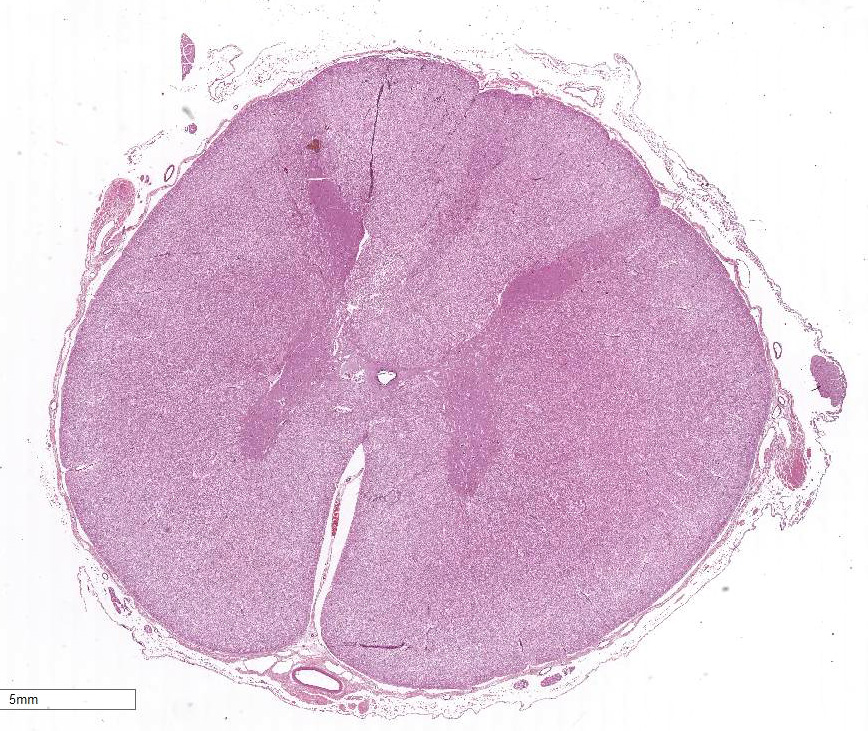
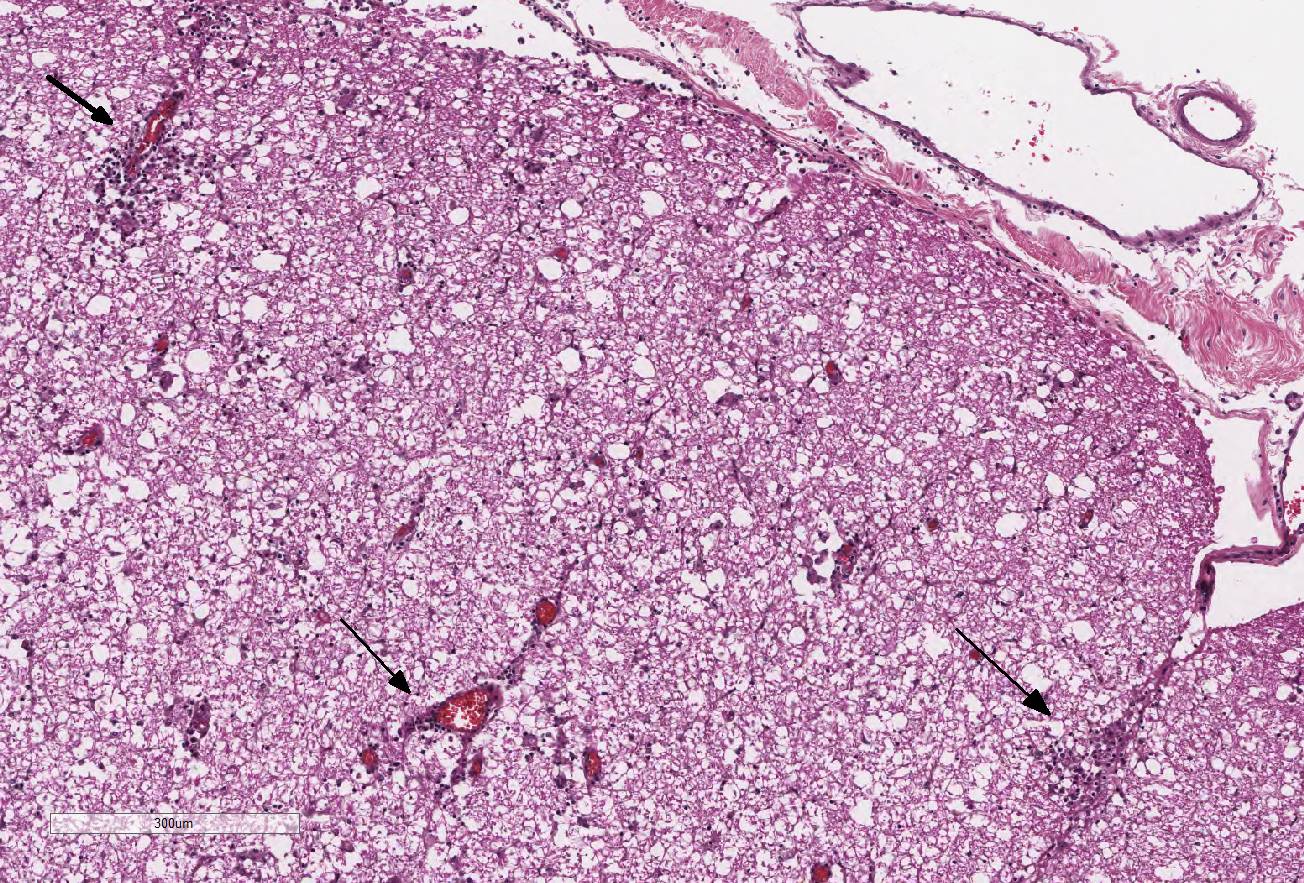
Extending from just caudal of C1 to C5, there is a locally
extensive area of rarefaction and multifocal to coalescing accumulations of
glial cells and gitter cells, unilaterally involving the dorsal funiculus at
the level of the gracile and cuneate fasciculi. Spinal cord inflammation is
most concentrated at C3 and includes significant perivascular cuffing, few to
moderate lymphocytes and plasma cells and scattered eosinophils. The associated
spinal gray column is unilaterally affected with similar inflammation in these
sections. Numerous swollen axons (spheroids) are present at C2 and C3 spinal
cord sections. At the level of C2, there are occasional glial nodules in the
contralateral dorsal funiculus as well. Small numbers of lymphocytes and plasma
cells are diffusely present in the meninges and are more concentrated over the
dorsolateral funiculus. The dorsal spinal nerve root ganglia are infiltrated
with small numbers of lympho-cytes and plasma cells at the level of C2 and C3.
Cervical spinal cord (C1-C5): Meningo-myelitis,
nonsuppurative and eosinophilic, unilateral, focally extensive, severe with
spheroid formation, cervical spinal cord, dorsal funiculus.
Condition:
Myelomalacia
Contributor Comment:
The gross and histopathologic findings in this case are highly
suggestive of cervical stenotic myelopathy. The unilateral distribution of the
lesions coincides with the focus of stenosis observed in the spinal canal.
Microscopic changes in the spinal cord include rarefaction, accumulations of
glial and gitter cells, and lymphocytes, plasma cells, and scattered
eosinophils. The horse, in this case, was 17 years old, considerably older
than the typical case of cervical vertebral stenotic myelopathy (8-18 months
and 1-4 years of age). However, several retrospective studies have documented
this condition in horses up to 22 years of age.
3,4
Cervical
vertebral stenotic myelopathy, commonly referred to as Wobblers syndrome, is
characterized by lesions in the spinal cord caused by narrowing of the spinal
canal or compression by the vertebral articular processes.
6,7 There
are two pathological syndromes: cervical vertebral instability (CVI) and
cervical static stenosis (CSS). Clinical signs for both pathological syndromes
include ataxia with the hindlimbs more commonly and more severely affected than
the forelimbs.
7 Cervical vertebral instability is characterized by
the narrowing of the spinal cord when the neck is ventroflexed. The cranial
articular process of the vertebral bodies project in a ventro-medial direction
and impinge on the spinal cord. C-3 to C-5 of the spinal cord is the most
affected area.
6 Young, rapidly growing horses between the ages of
8-18 months are predisposed to this condition. Breed dispositions include
Thoroughbreds and Quarter horses, and males are affected more often than
females. Contributing factors may be
ad libitum feeding of high-energy
and high-protein diet as well as copper deficiency.
6,7
Cervical
static stenosis is the less common syndrome. It is characterized by the
compression of the spinal cord at the level of C-5 to C-7 due to the thickening
of the ligamentum flavum and the dorsal laminae of the vertebral arches.
6
Predispositions are similar to those seen in CVI except horses aged one to four
years are commonly affected.
3 The position of the neck does not
determine whether or not the chord is compressed.
JPC Diagnosis:
Spinal cord, dorsal medial fasciculi: Necrosis, focally extensive,
asymmetric with lymphohistiocytic and eosinophilic perivasculitis and lepto-meningitis,
quarterhorse,
Equus caballus.
Conference Comment:
This interesting case generated spirited discussion amongst
conference participants. While attendees essentially agreed with the
contributors histopathologic description and morphologic diagnosis, there was
no consensus for the histogenesis of the necrotizing lesion in the spinal cord
of this horse. The conference moderator offered an alternative inter-pretation
of an infectious cause, with Sarcocystis neurona causing acute onset
weakness, ataxia, and a focally extensive area of necrosis in the dorsal medial
spinal cord with corresponding lymphohistiocytic and eosinophilic
leptomeningitis. The conspicuous eosinophilic component of the perivasculitis
and leptomeningitis in this case, may suggest a parasitic etiology.
S. neurona is an apicomplexan protozoan parasite which causes equine
protozoal myeloencephalitis (EPM), a relatively common and severe neurologic
disease in horses. Opossums are the definitive host for the parasite and spread
the disease by fecal shedding of sporocysts into the environment.1
Unfortunately, no api-complexan schizonts or merozoites were observed in any
examined tissue sections. Other potential etiologies offered by conference
participants included acute intervertebral disc rupture and fibro-cartilaginous
embolism, although disk material was not visualized within the section.
As noted by the contributor,
the age of the horse (17-years-old) is a highly atypical presentation for both
cervical stenotic myelopathy and cervical static stenosis.2,3,5
While most cases of cervical stenotic myelopathy involve ventral compression of
the spinal cord and spinal nerves with Wallerian-type degeneration of the white
matter of the dorsal and ventrolateral spinal cord affecting the descending
spino-cerebellar tracts of both the pelvic and thoracic limbs,2,5 in
this section, the ventral and lateral spinal cord is relatively unaffected.
The lesions seen in the submitted section of spinal cord are primarily located
in the dorsomedial spinal cord at the level of the fasciculus gracilus and
fasciculus cuneatus.
Some conference
participants noted occasional scattered 2x5 um filamentous bacilli multifocally
throughout the neuro-parenchyma. The brain and spinal cord are exquisitely
susceptible to post-mortem autolysis and putrefaction. As a result, the
conference moderator cautioned attendees against overinterpreting artifactual
bacterial overgrowth within post-mortem tissue samples of the central nervous
system, especially when they are not associated with inflammation, as in this
case.
References:
1. Bowman
DD. Protozoans. In: Georgis Parasitology for Veterinarians. 9th
ed. St. Louis, MO: Saunders Elsevier; 2009:104-105.
2. Janes JG, Garrett KS,
McQuerry KJ, et al. Cervical vertebral lesions in equine stenotic myelopathy. Vet
Pathol. 2015; 52:919-927.
3. Levine JM, Adam E, MacKay RJ, et al.
Confirmed and presumptive cervical compression myelopathy in older horses: A
retrospective study (1992-2004). J Vet Intern Med. 2007; 21:812-819.
4. Levine JM, Scrivani PV, Divers TJ, et
al. Multicenter case-control study of signalment, diagnostic features, and
outcome associated with cervical vertebral malformation-malarticulation in
horses. J Am Vet Med Assoc. 2010; 237(7):812-822.
5. Reed
SM. Cervical vertebral stenotic myelopathy: Pathogenesis. Proceedings of the
International Equine Neurology conference. College of Veterinary Medicine,
Cornell University, New York. 1997:45-49.
6. Thompson K. Bones and joints. In: Pathology
of Domestic Animals, 5th Edition. Edinburgh : Saunders; 2007:
44-46.
7. Zachary JF, McGavin DM. Nervous system. In: Pathologic Basis
of Veterinary Disease. 5th ed. St. Louis, MO: Mosby; 2012:816, 833-835.