Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 18
30 January, 2019
CASE III: 1013/15 (JPC 4085103)
Signalment: 18-year-old, female, bird, blue-eyed cockatoo (Cacatua sanguinea)
History: Bird suddenly presented respiratory distress and apathy, culminating in death few hours later. The bird was born in a house where it lived for seven years, and then moved to a small zoo where it lived for 11 years. This blue-eyed cockatoo shared premises with other bird species and, eventually, was transferred to a visiting area inside another aviary. The daily diet was a commercial ration (MegaZoo, Vale Verde, Minas Gerais, Brazil) and varied tropical fruits. Febendazole (4%) diluted in water or oral administration of ivermectin and albendazol was given once a year.
Gross Pathology: Grossly, lungs were markedly hyperemic and edematous. Visceral pleura was whitish and mildly thickened. The pericardial space and coelomic cavity contained small amount of a transparent fluid. In addition, there were moderate splenomegaly and multifocal black foci in the ovary.
Laboratory Results: None.
Microscopic Description:
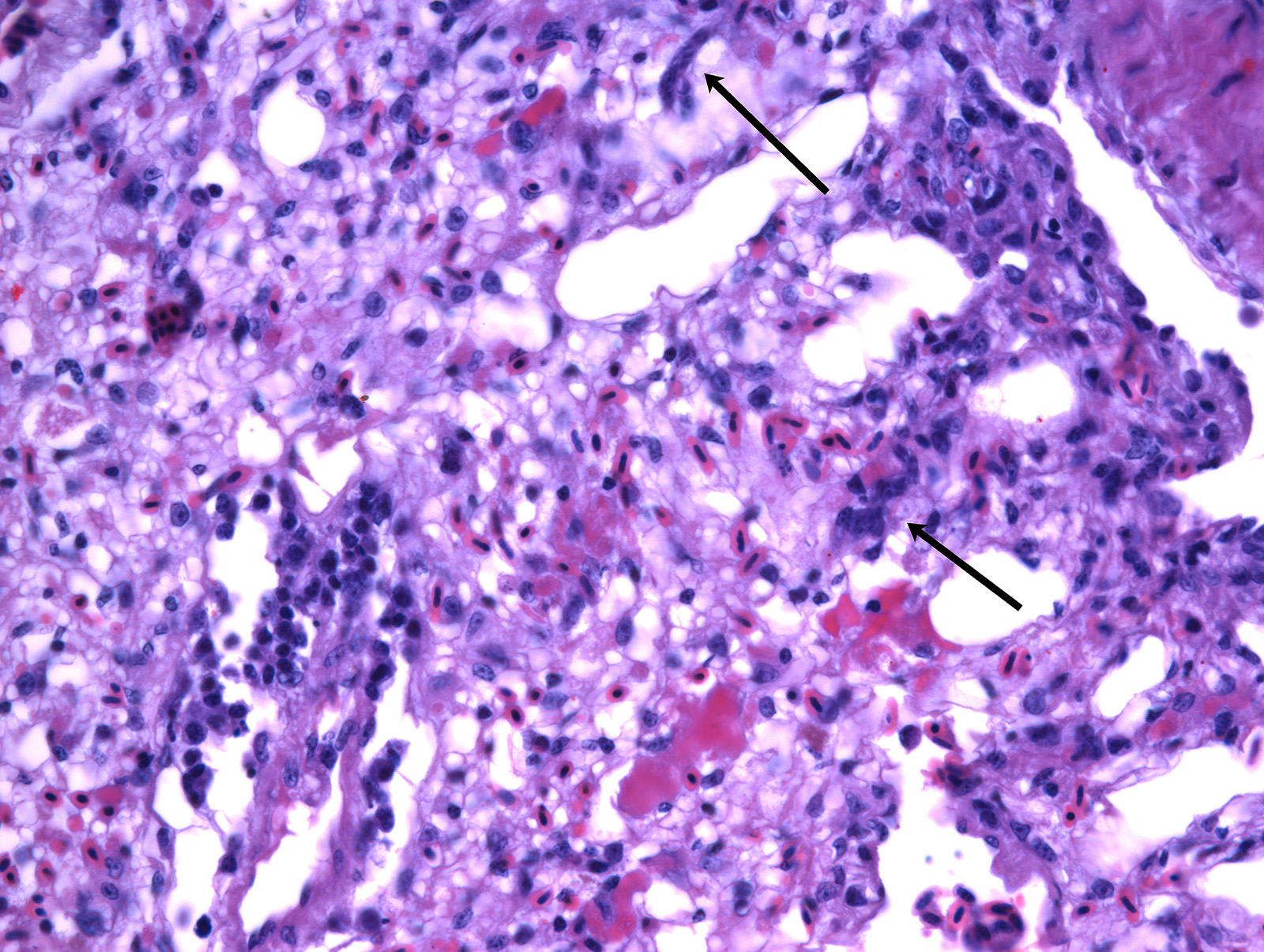
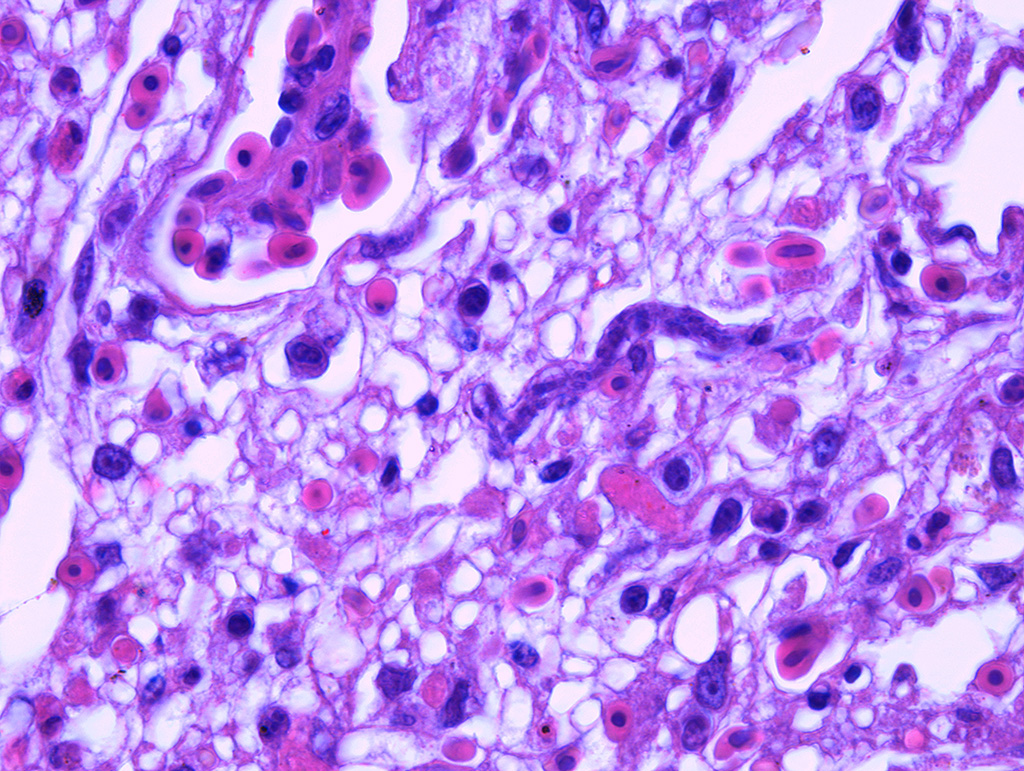
In the lungs, there was marked hyperemia and air spaces contained homogenous to fibrillar eosinophilic material. In some sections there were many erythrocytes within parabronchi. Cellularity was increased throughout the lungs, resulting in mild thickening of air capillary walls. In several perivascular areas there was mild to moderate infiltration of lymphocytes, histiocytes and few plasma cells. There was multifocal micro-thrombosis within capillaries. In some sections, lymphocytes, histiocytes and fewer plasma cells were also seen in the interstitium. Associate to these lesions, there were many sinuous schizonts lining the periphery of the capillaries. These schizonts were elongated, and ranged from 15 to 20 µm in length and from about 7 to 10 µm in cross-sectional diameter. There were some basophilic mature free merozoites within capillary lumen, which were most evident on the cross-sections.
Other organs (not submitted): in the heart there was mild to moderate, multifocal perivascular infiltration of lymphocytes and plasma cells. The liver had moderate to marked increase in the cellularity due to accumulation of histiocytes, lymphocytes and plasma cells in the sinusoids. In the kidneys, there was multifocal, mild interstitial and perivascular infiltration of lymphocytes and plasma cells. In the spleen, there was mild increased in the differentiation of lymphocytes to plasma cells. In the ovary, there was multifocal melanosis.
Contributor’s Morphologic Diagnoses:
Lungs: pneumonia interstitial lymphoplasmacytic acute, diffuse, moderate, with protozoa morphologically compatible with Sarcocystis falcatula.
Contributor’s Comment: Clinical history, gross and histologic lesions associated with sinuous schizonts in the lungs, suggests that this bird had fatal pulmonary sarcocystosis, presumed to be due to Sarcocystis falcatula.
Sarcocystis falcatula utilizes two-host life cycle. The only known definitive host in North America is the opossum (Didelphis virginiana).12 South American opossums (D. marsupialis and D. albiventris) can be the definitive hosts for S. falcatula, and S. falcatula-like protozoans.2,3 The aviaries where this bird was kept were enclosed by wire mesh on all sides, including the roof and the contact with feces of wilds animals probably occurred. The wild opossum (Didelphis albiventris) inhabits the forest surrounding the area of the small zoo, and is frequently seen at night in the area near the enclosures.
Asexual reproduction occurs in the intermediate host and is characterized by schizogony (merogony) and formation of sarcocysts in skeletal muscle. Sarcocystis falcatula can use a large variety of bird species as intermediate hosts, including Passeriformes,9 Psittaciformes,1,8 and Columbiformes.5,16 S. falcatula is highly pathogenic to intermediate hosts, especially to psittacines, because of its prolonged schizogony (5 months or more) and its fatal pulmonary presentation with many immature schizonts.1,3,7 The merogony phase in the intermediate hosts takes place in arteries, capillaries, veins, and venules of lungs, liver, kidney and brain.12,13 Ultimately, the merozoites give rise to sarcocysts in striated muscle. Then, mature sarcocysts can be found in cardiac and skeletal muscles.13
Clinical signs of acute fatal pulmonary sarcocystosis include severe dyspnea, anorexia, lethargy and loss of weight prior to death.12,16 Pulmonary sarcocystosis was described in different species of birds such as pigeons16, cockatiels1, ring-necked parakeets and African grey parrots5. Like this blue-eyed cockatoo, similar clinical presentations and lesions are usually found in other captive birds of Psittacidae family (Psittacinae and Arinae subfamily). Mature sarcocysts in the pectoral muscles were found in a free ranged macaw (Anodorhynchus hyacinthinus) and in a dusky parrot (Pionus fuscus) necropsied in our lab.
Pulmonary lesions were the most prominent finding in this bird and, according to Smith et al.12,13 are directly related to the pathogenesis of Sarcocystis infection, because endothelial cell invasion by schizonts occurs during asexual multiplication. The schizogony of this protozoan parasite in all bird species usually begins in the endothelium of capillaries and venules in the lamina propria of the small intestine.7 In parrots and parakeets, pulmonary schizogony is more intense than in other organs. In contrast, schizogony in pigeons is more intense in the liver.14,16 There are two hypotheses for these differences: the host cellular immune response for controlling Sarcocystis infection differs among bird species15, the type of host cell infected influencing the growth and persistence of S. falcatula merozoites6 and amount of sporocysts ingested by a bird7. Pulmonary hyperemia and edema result from stenosis of the capillaries and venules caused by the protozoan parasite, in addition to microthrombi formation induced by endothelial lesions during schizogony.12
The species of Sarcocystis that infected the bird in the study could not be determined and awaits further study. Sarcocystosis should be considered in the differential diagnosis of sudden death and pneumonia in captive birds. The usual occurrence of sarcocystosis is probably associated with the frequency of the definitive host and the close contact between birds and these animals.
Contributing Institution:
Veterinary School, Universidade Federal de Minas Gerais – www.vet.ufmg.br
JPC Diagnosis: Lung: Pneumonia, interstitial, histiocytic, diffuse, moderate with mild multifocal necrosis, and occasional intraendothelial apicomplexan meronts.
JPC Comment: The contributor has provided an excellent review of the pathogenesis of Sarcocystis falcatula infection in the bird. The recent literature contains a number of articles on the isolation of this parasite from a number of species of its definitive host (opossums native to various regions of the world), as well as details of the various manifestations of infection in a wide range of avian species, such as the recent report of necrotizing meningoencephalitis in a bare-faced ibis.8
Historically, S. falcatula was named by Stiles in 1893 from a specimen of a rose-breasted grosbeak. The parasite’s life cycle (similar to other sarcocysts, utilizing herbivores as intermediate hosts and carnivores as definitive hosts) and potential for infecting a wide variety of avian species was determined by Box and Smith in 1982. They determined that S. falcatula utilized only avian species as intermediate hosts and were not infective for poultry. Until 1995, it was believed that S. falcatula was the only sarcocyst utilizing the Virginia opossum (Didelphis virginianus) as its definitive host, until the discovery in 1995 of Sarcocystis neurona by Dubey and Lindsey, another agent which causes potentially fatal disease in intermediate hosts.4
Recently Dubey et. Al. have posited that there may be more than one particular species of Sarcocystis that cycles between avian species and opossums, suggesting than many of the earlier reports of S. falcatula in which molecular or immunohistochemical identification of the parasite was performed may be in question.4 In addition, this report discusses the potential immunohistochemical cross-reaction between the schizonts of S. falcatula and S. neurona based on the stage of the parasite used for immunization, method of preparation of zoites, stage of the parasite in test tissues, and individual variability among immunized rabbits.4 According to Dubey, a god in this field (and for whom Sarcocystis jaypeedubeyi is phonetically named), the structure of the sarcocyst wall (best viewed by ultrastructure) is the most reliable way to differentiate between two species of Sarcocystis, especially within the same host species.4
References:
- Clubb SL, Frenkel JK. Sarcocystis falcatula of opossums: transmission by cockroaches with fatal pulmonary disease in psittacine birds. J Parasitol. 1992;78:116–124.
- Dubey JP, Venturini L, Venturini C, Basso W, Unzaga J. Isolation of Sarcocystis falcatula from the South American opossum (Didelphis albiventris) from Argentina. Vet Parasitol. 1999;86:239–244.
- Dubey JP, Lindsay DS, Rezende PCB, Costa AJ. Characterization of an unidentified Sarcocystis falcatula-like parasite from the South American opossum, Didelphis albiventris from Brazil. J Eukaryot Microbiol. 2000;47:538–544.
- Dubey JP, Garner MM, Stetter MD, Marsh AE, Barr BC. Acute sarcocystic falcatula-like infection in a carmne bee eater and immunohistochemical cross-rectivity between Sarcocystis falcatula and Sarcocystis neurona. J Parasitol 2001; 87(4):824-832.
- Ecco R, Luppi MM, Malta MC, Araújo MR, Guedes RM, Shivaprasad HL. An outbreak of sarcocystosis in psittacines and a pigeon in a zoological collection in Brazil. Avian Dis. 2008;52:706-710.
- Elsheikha HM, Saeed MA, Fitzgerald SD, Murphy AJ, Mansfield LS. Effects of temperature and host cell type on the in vitro growth and development of Sarcocystis falcatula. Parasitol Res. 2003;91:22–26.
- Hemenway MP, Avery ML, Ginn PE, Schaack S, Dame JB, Greiner EC. Influence of size of sporocyst inoculum upon the size and number of sarcocysts of Sarcocystis falcatula which develop in the brownheaded cowbird. Vet. Parasitol. 2001;95:321–326.
- Jacobson ER, Gardner CH, Nicholson A, Page CD. Sarcocystosis encephalitis in a cockatiel. J. Am. Vet. Med. Assoc. 1984;185:94–96.
- Konradt G, Bianhi MV, Leite-Filho RV, da Silva BZ, Soares RM, Pavarini SP, Driemeier D. Necrotizing meningoencephalitis caused by Sarcocystis falcatula in a bare faced ibis. Parasitol Res 2017 116(2):809-812.
- Luznar SL, Avery ML, Dame JB, Mackay RJ, Greiner EC. Development of Sarcocystis falcatula in its intermediate host, the brown-headed cowbird (Molothrus ater). Vet. Parasitol. 2001;95:327–334.
- Neill PJG, Smith JH, Box ED. Pathogenesis of Sarcocystis falcatula (Apicomplexa: Sarcocystidae) in the budgerigar (Melopsittacus undulatus), IV. Ultrastructure of developing, mature and degenerating sarcocystis. J. Protozool. 1989;36:430–437.
- Smith JH, Meier JL, Neill PJG, Box ED. Pathogenesis of Sarcocystis falcatula in the budgerigar, I. Early pulmonary schizogony. Lab. Invest. 1987a;56:60–71..
- Smith JH, Meier JL, Neill PJG, Box ED.. Pathogenesis of Sarcocystis falcatula in the budgerigar, II. Pulmonary pathology. Lab Invest. 1987b;56:72–84.
- Smith JH, Neill PJG, Box ED. Pathogenesis of Sarcocystis falcatula (Apicomplexa: Sarcocystidae) in the budgerigar (Melopsittacus undulatus), III. Pathologic and quantitative parasitologic analysis of extrapulmonary disease. J. Parasitol. 1989;75:270–287.
- Smith JH, Neill PJ, Dillard EAI, Box ED. Pathology of experimental Sarcocystis falcatula infections of canaries (Serinus canaries) and pigeons (Columba livia). J Parasitol. 1990;79:59–68.
- Suedmeyer WK, Bermudez A, Barr BC, Marsh AE. Acute pulmonary Sarcocystis falcatula-like infection in three Victoria crowned pigeons (Goura Victoria) housed indoors. J Zoo Wild Med. 2001;32:252–256.