Signalment:
Gross Description:
Histopathologic Description:
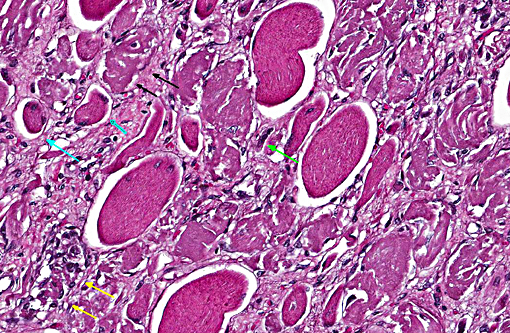
There is extensive loss of myofibers with replacement by variably dense fibrous connective tissue that expands the epimysium and the perimysium. This fibrous connective tissue occasionally contains rare scattered macrophages and lymphocytes, but active inflammation is mild to absent overall. There are frequent, scattered hemosiderin-laden macrophages. Frequently surrounding or within areas of fibrosis there are multifocal elongated, narrow myocytes with lightly basophilic cytoplasm containing multiple centralized nuclei arranged in a row (strap cells).
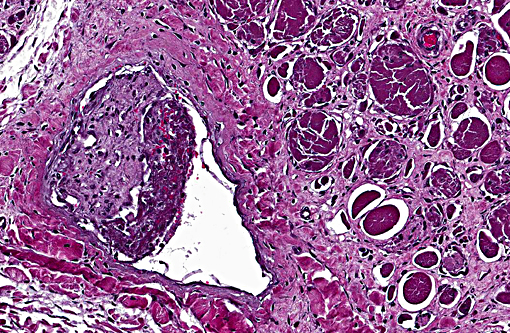
Multiple vessels, particularly small and medium caliber arterioles (but also some large veins) contain fibrin thrombi. Most of these thrombi are older and embedded in circumferential, proliferative spindle-shaped cells with foamy cytoplasm. This proliferation occurs in one to all layers of affected vessels and occludes the lumen, but there are small capillaries present with some red blood cells (recanalization). There is occasionally perivascular hemorrhage. Peripheral nerve bundles within the fascial planes tend to have the epineurium expanded by prominent myxomatous matrix.
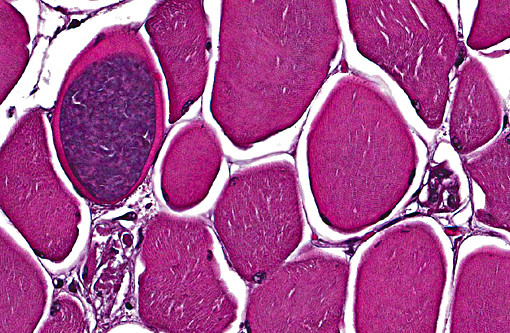
Some slides contain myofibers expanded by well-demarcated, ovoid cysts filled with innumerable basophilic organisms (Sarcocystis species, suspected) that are not associated with inflammation.
Morphologic Diagnosis:
Skeletal muscle: Locally extensive multiphasic myocyte degeneration, regeneration, necrosis, and loss with extensive fibrosis.
Small to medium caliber veins and arteries: Multifocal fibrin thrombi with transmural spindle cell proliferation and recanalization.
Condition:
Contributor Comment:
Microscopically, the myocyte degeneration and regeneration in the lesion is multiphasic. This is not consistent with a traditional capture myopathy resulting from a single incident. Capture myopathy is thought to result from a combination of local hyperthermia and lactic acidosis coupled with altered blood flow due to massive catecholamine release when an animal (particularly a non-domesticated animal) struggles while being restrained. The resulting combination of hyperthermia, acidosis, and altered blood flow results in localized myonecrosis that can produce a number of distinct syndromes depending on the duration of stress, the extent of the lesion, and the nature of recuperation.(2)
This lesion is problematic with regards to capture myopathy for two reasons: a case with a single reported capture event should produce a monophasic myonecrosis and vascular lesions are not considered a consistent aspect of capture myopathy. Upon extensive discussion among pathologists in the diagnostic laboratory, we felt that a possible explanation for the observed lesions was an initial incidence of capture myopathy or trauma (or any other possible reason for the initial bout of recumbency). Following the animal being recumbent, and perhaps due an initial exertional rhabdomyolysis, the pressure of the animals weight on the limb likely produced a localized pressure ischemia.
Shifting of the animals weight could have produced various incidences of ischemia and reperfusion over the course of disease, resulting in multiple incidences of muscle damage over time, which could explain the multiphasic nature of the myocyte degeneration and regeneration. The vascular lesions could indicate that the initiating injury could have been an infarct, but in the absence of a source of inflammation or thrombosis anywhere else in the body, it seems more likely that the proliferative and thrombotic vascular lesions are secondary to a combination of pressure ischemia and altered blood flow. Of course, the development of thrombi due to stagnation of blood in the vessels likely resulted in infarcts later on in the course of disease, adding to the ischemic lesions produced by pressure on the limb.
Another interesting aspect of this case was the absence of an active pigmentary nephrosis in this animal. Although there were a few chronic infarcts in the kidneys along with a few dilated tubules, there were no pigment casts present in the tubules. Despite the fact that the lesions in the limb were likely ongoing, one can speculate that the myocyte degeneration in the limb was not severe enough to produce an active myoglobinurea.
JPC Diagnosis:
1. Skeletal muscle: Degeneration, necrosis and atrophy, diffuse, severe, with regeneration.
2. Skeletal muscle: Sarcocysts, multiple.
3. Skeletal muscle, vessels: Thrombosis, acute and chronic, multifocal, moderate.
Conference Comment:
The size of the vessels and duration of obstruction play important roles in determining the size and nature of vascular-origin myofiber necrosis. Blockage of smaller capillaries results in less severe injury due to abundant vascular anastomoses, but can cause segmental myofiber necrosis along with regeneration and, if the cause is ongoing, the lesion would be polyphasic. This is in contrast to blockage of a large artery, where a large section of ischemic muscle would present as acutely necrotic and as a monophasic lesion, and which would eventually be replaced by fibrosis.(3) Causes of muscle ischemia and necrosis that may apply in this case include external muscle pressure and vascular obstruction (due to being recumbent), and swelling of a muscle confined within non-expandable fascia (i.e. compartment syndrome). Additionally, reperfusion injury can result when the animal is moved, which may also cause myofiber necrosis. Muscular injuries in downer cows can be either monophasic or polyphasic depending on duration of recumbency.(3)
Following myocyte necrosis, remaining myofiber nuclei are unable to divide and rely on activation of adjacent satellite cells, which are more resistant to injury than myocytes. These cells divide and become activated myoblasts in order to regenerate the damaged segment of muscle. During the regenerative process, myoblast nuclei can be seen within the center of regenerating myofibers, which is a key finding indicative of regeneration. Success of regeneration depends on whether the basal lamina is intact and whether viable satellite cells are present. In the case of significant muscle trauma, there is often disruption of the basal lamina and most healing will, therefore, occur by fibrosis.(3)
Upon consultation, JPC physician neuromuscular pathologists did not believe there was a vascular or neurogenic contribution to the histologic changes.
There was slide variation with some sections having low numbers of sarcocysts which contained numerous bradyzoites. Participants agreed that the presence of the sarcocysts did not contribute to the muscular lesion.
References:
1. Stussy SJ, Findholt SL, Johnson BK, Noyes JH. Selenium levels and productivity in three Oregon elk herds. Northwest Science. 74:2. 2000. 97-101.
2. Spraker TR. Stress and capture myopathy in artiodactylids. In Zoo and Wild Animal Medicine: Current Therapy 3. Fowler ME, ed. WB Saunders Company. 1993. 481-488.
3. Valentine BA, McGavin DM. Skeletal muscle. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012:879-898.