Signalment:
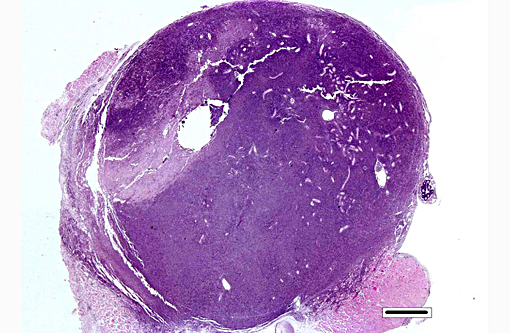
Gross Description:
Histopathologic Description:
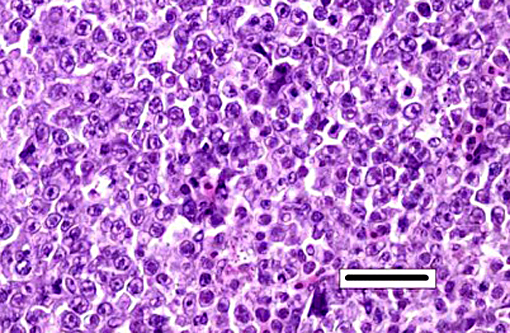
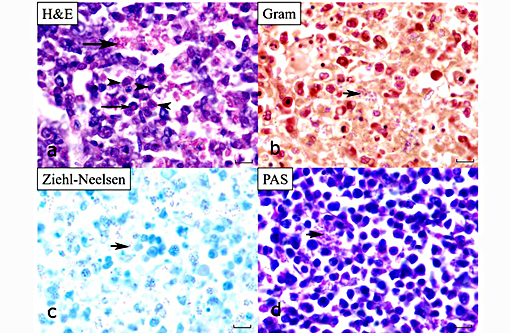
Kidney: Hematopoietic kidney is massively expanded and normal architecture effaced by densely cellular sheets of mononuclear round cells, whose morphology is suggestive of lymphoid/lymphoblastic origin. There is no intervening stroma, but small numbers of remnant tubules are present within the cell sheets. A subpopulation of cells have condensed aggregations of multiple (typically around 4 to 8) pale eosinophilic faintly refractile circular to ovoid spores (each approximately 1 x 3 μm) contained within their nuclear membranes (Figure 3a), and more numerous cells with intranuclear eosinophilic vacuoles/cytoplasmic folds are present. Varying numbers of spores are present in the extracellular milieu as a result of nuclear/cellular rupture, with phagocytosis by intermingled macrophages. Spores are most abundant in small multifocal areas of necrosis. There is extension of lymphoblastoid infiltrates into paravertebral striated muscle groups and connective tissue, spinal ganglia and spinal cord meninges. Similar cells are also present within the lumina of blood vessels. Spores stain weakly gram-positive, focally Ziehl-Neelsen positive and weakly PAS-positive.
Morphologic Diagnosis:
Lab Results:
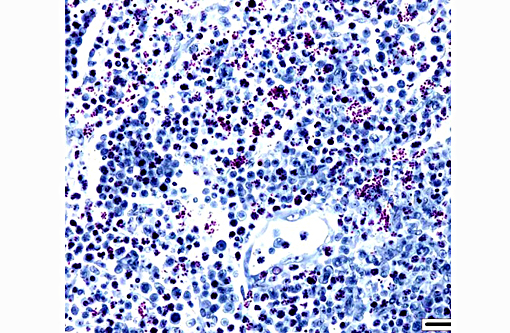
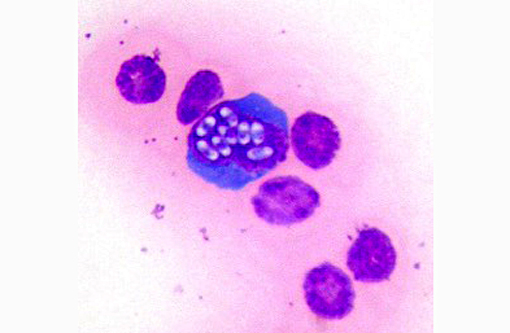
Cytologic description: Blood smear: Significant artifactual changes are present, resulting in poor cytoplasmic preservation of red cells, and abnormal chromatin staining. Assessment of red cell parameters is unreliable for this reason. This fish has a significant monocytosis, and lymphocytes also appear increased in number. In addition, significant numbers of the monocytic cells, and occasional presumptive lymphocytes, have intranuclear clusters of multiple (regularly 8 in some cells, occasionally more) elliptical refractile spores, consistent with intranuclear microsporidia.
Coelomic fluid: Moderately cellular smears in which mononuclear cell/macrophages and lymphocytes predominate. Cellular morphology is shrunken and nuclei are more condensed than in the blood smears. Intranuclear microsporidia are abundant within the mononuclear populations, and aggregates of similar spores within cytoplasmic fragments are also a feature
Condition:
Contributor Comment:
In the last 2 years in the United Kingdom we have recognized this infection as a significant cause of mortality in aquarium-bred colonies of lumpfish destined for public aquaria, and in research colonies used for experimental studies into lumpfish husbandry. Along with wrasse, lumpfish are increasingly employed as cleaner fish for the control of sea lice in Atlantic salmon aquaculture, and there is an increasing interest in their susceptibility to diseases, particularly those to which salmonids may potentially be susceptible.
On cytological examination, cells in the coelomic fluid and monocytes/lymphocytes within blood smears contain numerous distinct intranuclear microsporidial spores. They are easier to see in this context than in the more shrunken nuclei of histological sections. Enhanced visualization may have been possible with a Peterson-Luna stain.(8) In histological sections, infiltrates of proliferating lymphocyte-like cells with intranuclear organisms expand the hematopoietic kidney early in the course of the disease, before disseminating (consistent with the presence of spores in circulating monocytes in the blood smears) into multiple organs (including in advanced cases gastrointestinal tract, meninges, spleen, gonads and coelomic and cutaneous connective tissues). Sheets of infiltrating cells contain foci of necrosis and there is extracellular liberation of spores, which are frequently phagocytosed by admixed populations of reactive macrophages. The identity of the proliferating cells is difficult to determine with certainty based on morphology alone, but the most recent publication suggests that they are lymphocytes and monocytic precursor cells(2, 3) and the earlier report describes the affected cells as lymphocyte-like.(6)
Although formerly classified as protozoans, microsporidia are now considered to be more closely related to fungi. They are obligate intracellular spore-forming organisms with a pan-taxonomic distribution, and commonly infect fish, typically causing xenomas in a range of tissues depending on host and parasite(6). However, intranuclear microsporidia are also recognised in fish, in particular a closely-related parasite, Nucleospora (formerly Enterocytozoon) salmonis, which is distributed worldwide, and has been recorded in several salmon species, causing anemia and lymphoblastosis.(4) The precise mode of transmission of N. cyclopteri remains uncertain, but many microsporidia, including N. salmonis, have a direct life cycle. The possibility of vertical transmission has also been discussed.(2) In fish, intranuclear microsporidia have also been described infecting enterocytes in Tanzanian killifish (Nothobranchius rubripinnis) and, most recently, infecting enterocytes leading to a wasting syndrome in farmed gilthead sea bream (Sparus aurata).(7)
JPC Diagnosis:
Kidney, hematopoetic tissue: Necrosis, diffuse with marked hyperplasia, multifocal infarction, and intranuclear, intracytoplasmic and free microsporidia.
Kidney: Nephritis, histiocytic, diffuse, marked with tubular degeneration, necrosis and loss and intranuclear, intracytoplasmic and free microsporidia.
Conference Comment:
The expansion of hematopoetic tissue was striking in this case, characterized by sheets of large blastic cells reminiscent of a lymphoproliferative neoplasm, admixed with a second population of smaller phagocytic round cells. Two additional alternate diagnoses in this case were discussed: lymphoproliferative neoplasia and Exophiala sp. infection. Exophiala sp. fungal infections can cause disease in both freshwater and marine species including channel catfish, lake and cutthroat trout as well as Atlantic salmon and cod among others. Lesions can be both cutaneous and visceral with areas of necrosis and chronic granulomatous inflammation, and in some cases infection has a predilection for the kidney with the presence of renomegaly and involvement of other visceral organs.(9) Fungal infection characteristically results in lesions with vasculitis, thrombosis, and infarction.
References:
1. Alarc³n, M., Thoen, E., Poppe, T. T., Born, G., et al. Co-infection of Nucleospora cyclopteri (Microsporidia) and Kudoa islandica (Myxozoa) in farmed lumpfish, Cyclopterus lumpus L., in Norway: a case report. J Fish Dis. 2015, Apr 10; doi: 10.1111/jfd.12372. E pub ahead of print.
2. Freeman, M. A., Kasper, J. M., & Kristmundsson, . Nucleospora cyclopteri n. sp., an intranuclear microsporidian infecting wild lumpfish, Cyclopterus lumpus L., in Icelandic waters. Parasit Vectors. 2013; 6:49.
3. Freeman, M. A. & Kristmundsson, . Ultrastructure of Nucleospora cyclopteri, an intranuclear microsporidian affecting the Atlantic lumpfish (Cyclopterus lumpus L.). Bulletin of the European Association of Fish Pathologists. 2013; 33(6):194-198
4. Hedrick, R. P., Groff, J. M., & Baxa, D. V. Experimental infections with Enterocytozoon salmonis Chilmonczyk, Cox, Hedrick (Microsporea): an intranuclear microsporidium from chinook salmon Oncorhynchus tshawytscha. Dis Aquat Organ. 1991; 10:103-108.
5. Lom, J., & Dykova, I. Microsporidian xenomas in fish seen in wider perspective. Folia Parasitologia. 2005; 52:69-81.
6. Mullins, J. E., Powell, M., Speare, D. J., & Cawthorn, R. An intranuclear microsporidian in lumpfish Cyclopterus lumpus. Dis Aquat Organ. 1994; 20:7-13.
7. Palenzuela, O., Redondo, M. J., Cali, A., Takvorian, P. M., et al. A new intranuclear microsporidium, Enterospora nucleophila n. sp., causing an emaciative syndrome in a piscine host (Sparus aurata), prompts the redescription of the family Enterocytozoonidae. Int J Parasitol. 2014; 44:189-203.
8. Peterson TS, Spitsbergen JM, Feist SW, Kent ML. Luna stain, an improved selective stain for detection of microsporidian spores in histologic sections. Dis Aquat Organ. 2011; 95(2):175-80.
9. Sindermann CJ. Principal diseases of marine fish and shellfish 2nd ed. Vol. 1. San Diego: Academic press; 1990:71-72.