Signalment:
5-year-old male nyala (
Tragelaphus angasii). This animal was reported to be quiet and lethargic for several hours before being found dead by zoo staff. A clear brown, watery fluid was aspirated from the cardiac region by the referring veterinarian while attempting to take a post mortem blood sample.
Gross Description:
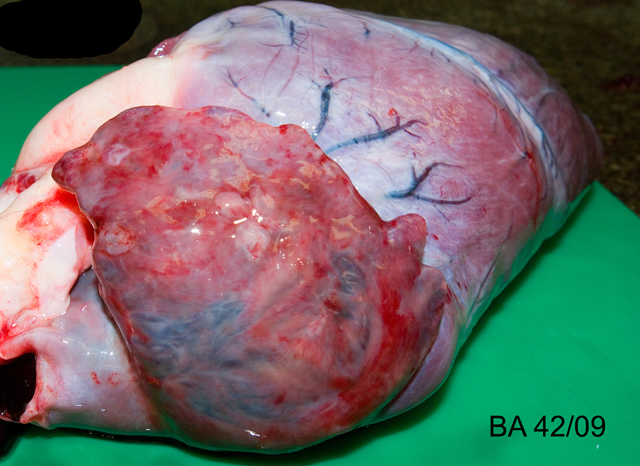
The carcass was in good nutritional condition. The pericardium contained approximately 40ml of red tinged watery fluid, and a cream to red, soft, smooth 8 x 0.5 x 0.2 cm clot of coagulated protein. The epicardium was multifocally mildly roughened to granular. The right atrium and atrial appendage were dilated and thin walled, and the right atrial appendage contained multifocal intramural nodules measuring 2-5mm in diameter which were firm and often had a mottled red to brown appearance, with a pale yellow / brown, firm to gritty central area (see Fig 1). The right atrial wall at the atrioventricular junction was mildly thickened and spongy. The heart weighed 560g, 0.66% of body weight (maximum normal for cow and goat is 0.66%).(6) The trachea and bronchi contained a moderate amount of white frothy fluid. The dorsal aspects of both lungs contained multifocal to coalescing regions of dark red discoloration (hemorrhage), which did not extend into the parenchyma. The liver had rounded borders, and oozed moderate amounts of blood from the cut surface. Skeletal muscles were macroscopically unremarkable.
Histopathologic Description:
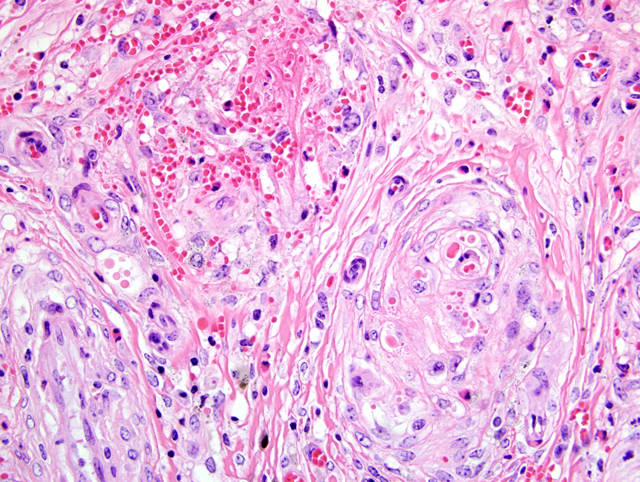
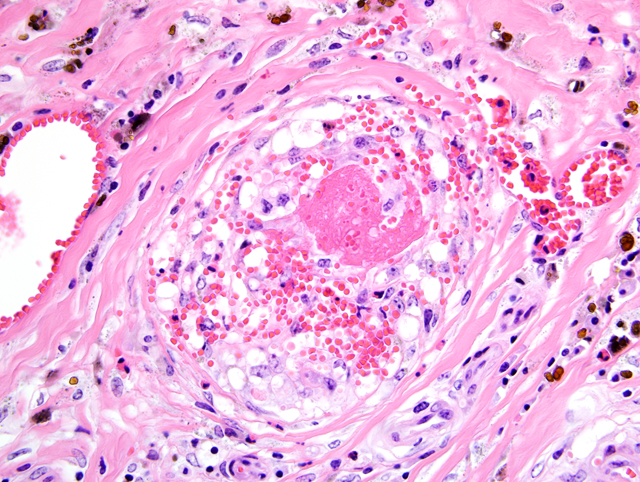
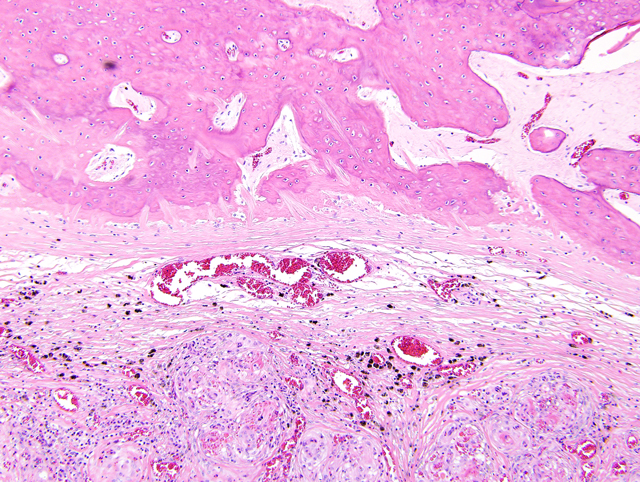
Right atrium, one section is examined. Regionally extensive and coalescing areas of the myocardium are replaced by loosely organized knots and whorls of plump spindloid cells (fibroblasts). There are also numerous clusters of small blood vessels in these areas (neovascularization). They are lined by plump endothelial cells, and the spindle cells tend to form circumferential rings around them. Occasional eosinophilic hyaline droplets are closely associated with the small vessels, interpreted to be plasma. The surrounding stroma and myocardium is infiltrated by large numbers of foamy macrophages, moderate numbers of pyknotic eosinophils, and smaller numbers of lymphocytes and neutrophils. There are also moderate to large numbers of extravasated red blood cells (hemorrhage). These regions are often surrounded by mature dense collagen bundles and moderate to large numbers of macrophages, which contain coarsely granular, brown pigment (hemosiderophages). Some blood vessels contain smudged eosinophilic material (fibrin thrombi). Occasional larger blood vessels contain moderate amounts of intramural smudged eosinophilic material (fibrin), creating a starburst appearance. These vessels are surrounded by a layer of foamy macrophages and fibroblasts, hemorrhage, pyknotic granulocytes, and lymphocytes (vascular fibrinoid necrosis). Myofibers in the remaining myocardium are occasionally shrunken, hypereosinophilic, and fragmented (myodegeneration). Moderate numbers of plasma cells and lymphocytes are scattered throughout the myocardium, together with moderate numbers of fibroblasts. Multifocal to coalescing regions of the myocardium are replaced or separated by finely granular, palely eosinophilic material (fibrosis). Multifocal areas contain irregular fragments of trabecular bone, and one fragment of bone has a paler central core which contains numerous large, pale blue grey cells within lacunae (chondrocytes, cartilage).
Morphologic Diagnosis:
Myocardial degeneration and loss, severe, multifocal to coalescing, with fibrinoid vasculitis, neovascularization, thrombosis, hemorrhage and osseous metaplasia - right atrial appendage, Nyala (
Tragelaphus angasi).
Lab Results:
Liver: vitamin E 34.22 μmol/kg FT (ref. range bovine = 3-18 μmol/kg); selenium 1.37 mg/kg DM (ref. range bovine: >23 mg/kg).
Condition:
Selenium deficiency
Contributor Comment:
Given the signalment, history, gross and histological findings, a diagnosis of nutritional myopathy due to vitamin E and/or selenium deficiency was considered to be most likely. Liu et al (1985) described histopathological features of nutritional myopathy in captive nyala, in particular noting coronary arteriolar fibrinoid necrosis in affected young animals.(4) An additional feature of this case is the striking neovascularization with small blood vessels surrounded by whorls of spindle cells. This is considered most likely to be a form of granulation tissue produced as part of the reparative process.Â
Vitamin E (as tocopherol) and enzymes containing selenium (glutathione peroxidase / glutathione reductase system) act as antagonists to free radicals and highly reactive unstable compounds produced either during normal cell function or as a result of disease or tissue injury.(8) Damage by free radicals is primarily caused by peroxidation of cellular and subcellular lipid membranes, resulting in loss of the ability to maintain essential differential ion gradients across the membrane. Free radicals may also cause damage to proteins of various cellular components, including mitochondria and endoplasmic reticulum.(1,2,4,8) Vitamin E and the selenium-containing glutathione enzymes act synergistically. However, they may remove different free radicals; therefore, a deficiency in either can lead to disease, with deficiencies in both leading to severe disease.(1,8) In this case hepatic vitamin E levels appeared to be adequate, whilst selenium levels were considered to be low.
Oxidative damage is commonly seen in actively contracting muscle fibers where damage to the cellular membrane allows influx of calcium ions, which are actively moved away from the calcium sensitive myofibers and stored within mitochondria. This results in a reduction in mitochondrial function and decreased energy production.(8) Calcium overload eventually causes a state of myofiber hypercontraction, causing degeneration of the myofilaments and coagulation of the contractile proteins.(8)
Nutritional myopathy (syn. white muscle disease, mulberry heart disease, nutritional myodegeneration, nutritional muscular dystrophy, stiff lamb disease) is recognized in many domestic and exotic species, most frequently associated with hypovitaminosis E and selenium deficiency. Reports in the nyala are common and it is considered to be a particularly susceptible species.(3-5,8) In domestic animals, nutritional myopathy is usually a disease of young animals, calves, lambs, swine and foals, with sporadic disease in adults. Affected young animals are usually well grown and thrifty, and affected adults are often those in good nutritional condition.(8) Factors related to vitamin E and selenium deficiencies may be rapid postnatal growth, poor quality feed, lush forage in heavily fertilized and watered pasture, selenium antagonists (e.g. copper, silver, zinc, sulphur), and grazing on dry pastures.(8)
JPC Diagnosis:
Heart, atrial appendage: Fibrinoid vascular necrosis and nodular proliferation, chronic-active, multifocal to coalescing, marked, with cardiomyocyte degeneration and loss, hemorrhage, fibrin thrombi, and fibro-osseous metaplasia.
Conference Comment:
Conference participants interpreted the striking vascular changes in this case as the primary lesion, with the myocardial changes occurring secondarily as reflected in the preferred morphologic diagnosis. However, an alternative view is that the myocardial degeneration and necrosis is unrelated to the vascular changes, as is supposed in pigs with mulberry heart disease. In the latter, arterioles of the heart, kidneys, liver, stomach, intestine, mesentery, skeletal muscle, and skin exhibit changes ranging from endothelial swelling, with increased permeability, to fibrinoid change with thrombosis and smooth muscle cell necrosis. A deficiency in vitamin E, rather than selenium, is now thought pivotal in the development of mulberry heart disease.(6) While tissues from other organs were not submitted for histopathologic evaluation in this case, finding a similar distribution of vascular lesions would be noteworthy. A list of selected diseases associated with vitamin E/selenium deficiency or imbalance in various animal species is available in
WSC 2008-2009, Conference 14, case III.
Conference participants considered a number of potential etiologies, including plant toxins (i.e. cardiac glycosides), an endotheliotropic virus, encephalomyocarditis virus, and malignant catarrhal fever. This case illustrates the importance of ancillary testing in a diagnostic setting, and conference participants discussed practical approaches to making the correct etiologic diagnosis in the field. While reserving fresh frozen tissues is ideal for analysis in cases of suspected toxicity or dietary deficiency, the conference moderator reminded participants it is feasible to quantify many minerals from formalin-fixed tissues. For cases of suspected selenium deficiency, when tissue samples are unavailable from the affected animal, viable alternatives include testing samples from herdmates (i.e. animal tissues, blood, and milk), soil, or forages and grains for selenium levels. In cases where animals have been administered parenteral or enteral supplemental selenium in response to a clinical suspicion of deficiency, blood and milk levels of selenium rise rapidly, obscuring the deficiency and precluding a diagnosis. Alternatively, measuring whole blood glutathione peroxidase (GSH-PX) is still useful because its enzymatic activity depends on incorporation of selenium into erythrocytes during erythropoiesis, which requires four to six weeks for enzyme levels to rise following selenium supplementation. By contrast, plasma GSH-PX activity, which does not rely on incorporation into erythrocytes, rises more quickly following supplementation.(7)
References:
1. Hill K, Motley A, Li X, May J, Burk R: Combined selenium and vitamin E deficiency causes fatal myopathy in guinea pigs. J. Nutr.Â
131:1798-1802, 2001
2. Kumar V, Abbas A, Fausto N: Cellular adaptations, cell injury, and cell death.Â
In: Robbins and Cotran Pathological Basis of Disease, eds. Kumar V, Abbas A, Fausto N, 7th ed, pp. 16-18. Elsevier Saunders, Philadelphia, PA, 2005
3. Liu SK, Dolensek EP, Herron AJ, Stover J, Doherty JG: Myopathy in the nyala. J Am Vet Med Assoc
181:1232-1236, 1982
4. Liu SK, Dolensek EP, Tappe JP: Cardiomyopathy and vitamin E deficiency in zoo animals and birds. Heart Vessels Suppl
1:288-293, 1985
5. Liu SK, Dolensek EP, Tappe JP, Stover J, Adams CR: Cardiomyopathy associated with vitamin E deficiency in seven gelada baboons. J Am Vet Med Assoc
185:1347-1350, 1984
6. Maxie MG, Robinson WF: Cardiovascular system.Â
In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 37-41. Elsevier Saunders, Philadelphia, PA, 2007
7. Radostits OM, Gay CC, Hinchcliff KW, Constable PD: Veterinary Medicine, A Textbook of the Diseases of Cattle, Horses, Sheep, and Goats, 10th ed., pp. 1746-1747. Saunders Elsevier, Philadelphia, PA, 2007
8. Van Vleet JF, Valentine BA: Muscle and tendon.Â
In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1., pp. 198-200, 236-242. Elsevier Saunders, Philadelphia, PA, 2007