WSC 2023-2024, Conference 24, Case 2
Signalment:
6-week-old male leopard tortoise (Stigmochelys pardalis).
History:
The animal presented with anorexia, lethargy, and yellow bumps along the skin. The animal was found deceased approximately 48 hours after onset of symptoms. There were 6 other clutch mates housed in the same enclosure with similar skin lesions, but without behavioral changes.
Gross Pathology:
The bilateral inguinal and gular skin is moderately thickened with fissures; ventrally and laterally, there are multifocal, pale tan-yellow, pinpoint, raised, nodular foci. No additional significant findings were observed.
Laboratory Results:
16S PCR and bidirectional Sanger sequencing identified the presence of Austwickia chelonae within the affected skin.
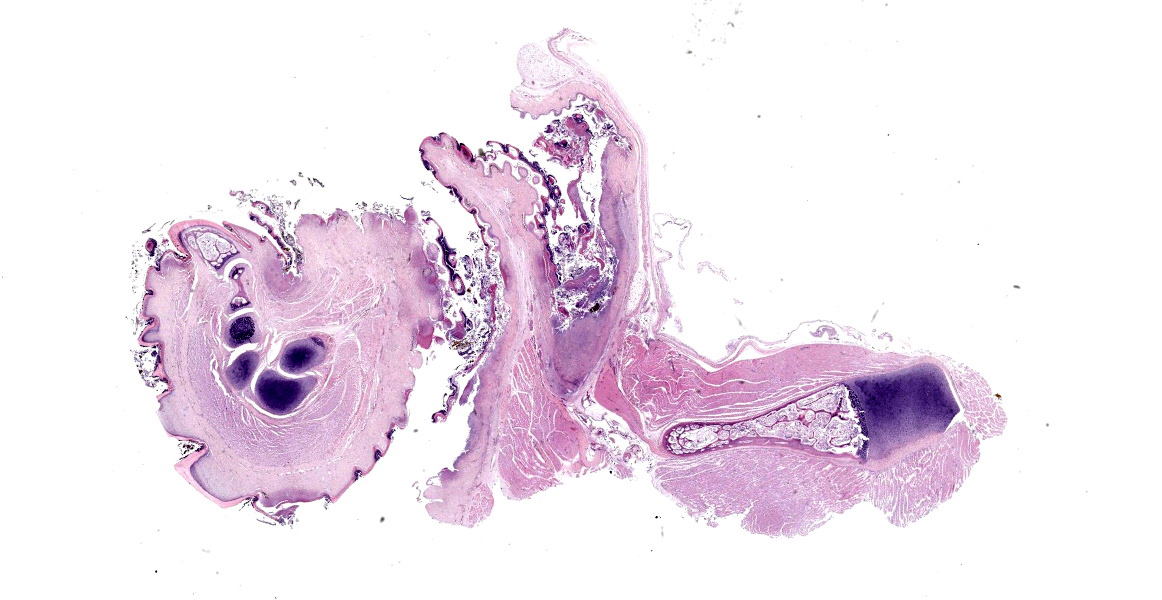
Microscopic Description:
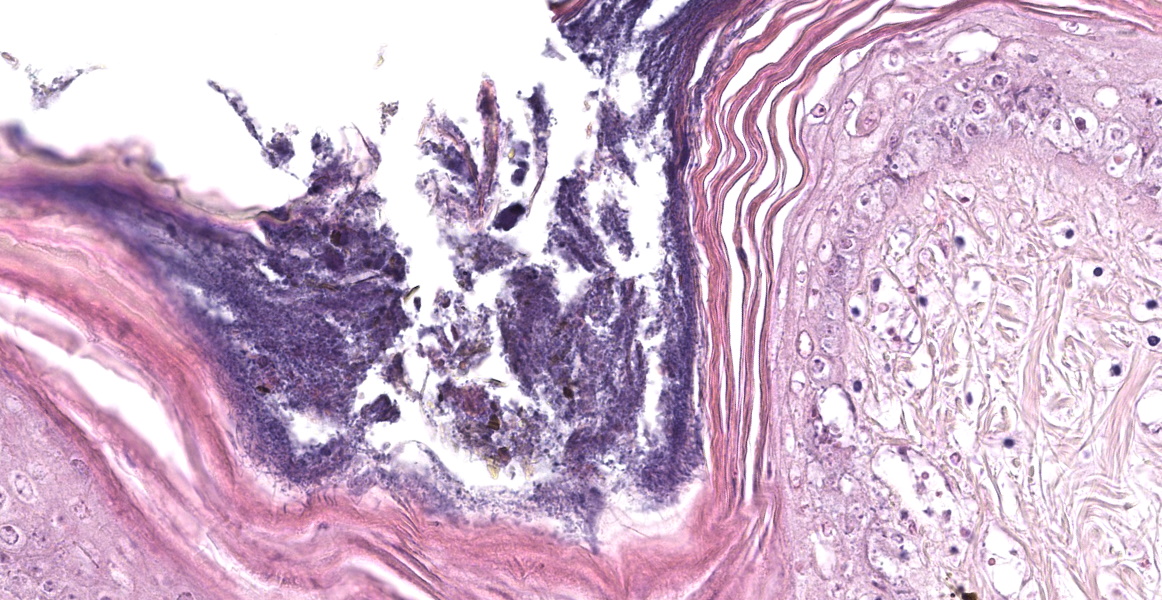
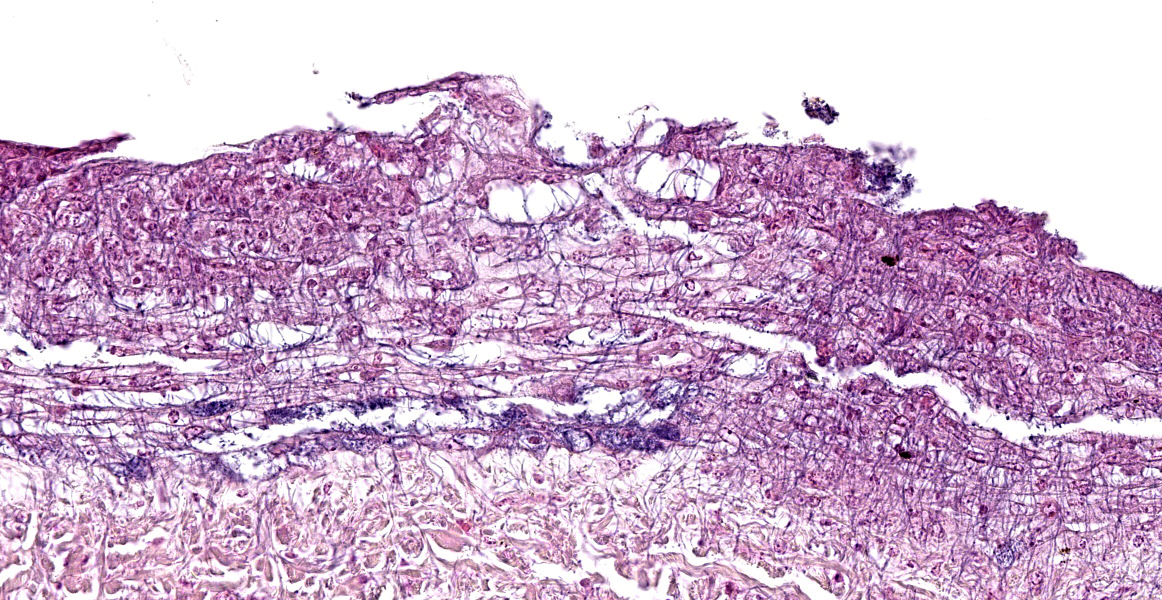
The epidermis of the distal hind limb is multifocally overlain by lamellar, compact keratin admixed with dense mats of primarily filamentous but also fewer coccoid bacteria. The filamentous bacteria, along with fewer cocci, invade into the dermis and superficial skeletal muscle. Multifocally, the epidermis is eroded to ulcerated with sloughing and epidermal-dermal junctional clefting of the extant epidermis. The affected epidermis is often homogenously eosinophilic with pale cellular outlines (coagulative necrosis) and contains variably prominent intracellular and intercellular vacuolation (edema). Similar regions of coagulative necrosis are present within the dermis with low numbers of extravasated erythrocytes, scattered granulocytes, and occasional histiocytes. Venules and arterioles in these regions are often disrupted and necrotic with invasion of filamentous bacteria transmurally into the vascular lumen. Similar findings are seen within the pericloacal, gular, and cephalic skin.
Contributor’s Morphologic Diagnosis:
Dermatitis, necrotizing, multifocal to coalescing, severe, chronic with abundant intralesional, gram-positive filamentous bacteria (morphology consistent with Austwickia chelonae) and fewer cocci.
Contributor’s Comment:
Both the gross and histologic findings in this leopard tortoise are consistent with the PCR identification of Austwickia chelonae. A. chelonae is a gram-positive, filamentous, facultative anaerobic actinobacterium that can cause disease (austwickiosis) in both captive and free-ranging reptiles. Prior to 2010, this organism was categorized within the same genus as perhaps the best known member of the Dermatophilaceae, Dermatophilus congolensis.3,5 First described in 1995 as Dermatophilus chelonae, the organism was later reassigned to the newly created genus, Austwickia (Family Dermatophilaceae).3,8 As part of its initial characterization using samples collected from captive chelonians in a western Australian Zoo, A. chelonae showed many unique features in comparison to D. congolensis. These included different biochemical characteristics, distinct morphology, and specific culture requirements, including optimal growth occurring at 27° C compared to 37° C for D. congolensis.8
Most commonly, gross findings in chelonids consist of dermatitis with hyperkeratosis and progressive epidermal necrosis and ulceration often resulting in the formation of yellow to white nodules that can extend into the subcutis.1,3 Although the extent of the potential host range of A. chelonae has not been fully determined, infections have been reported in numerous chelonian species, including captive desert (Gopherus agassizii), speckled cape (Homopus signatus), and sulcata (Centrochelys sulcata) tortoises.3,7,9 Less frequently, A. chelonae has been implicated as a cause of septic arthritis and visceral granulomas in captive tortoises.1,3 In captive, juvenile C. sulcata, A. chelonae infections were associated with extensive bone involvement with destruction of the nasal cavity and mandible.9 In 2022, A. chelonae was documented for the first time in free-ranging chelonians in North America as a cause of cutaneous granulocytic granulomas in a wild gopher tortoise (G. polyphemus).7
Though the exact reservoir of A. chelonae has not been determined, the organism has been isolated from environmental soil and water sources.4 It is suspected that initial local trauma to the skin, such as from tick bites or embedded foreign material, or inappropriate captive husbandry (high humidity or excessive soaking), are necessary to permit austwickiosis.7,9 A. chelonae has recently been documented to encode a gene for a toxin similar to diphtheria toxin that had been previously only described within the genus Corynebacterium.7 Diphtheria toxin terminates RNA synthesis and has been shown to notably interact with the host’s immune system.7 A presumptive diagnosis of austwickiosis may be made based on the combination of signalment combined with gross and histologic features using routine and special histochemical (Gram, GMS) stains. A definitive diagnosis of A. chelonae, however, requires PCR, culture, or metagenomic next-generation sequencing (mNGS).3,4
Austwickiosis is not restricted to chelonians. Captive inland bearded dragons (Pogona vitticeps) coinfected with A. chelonae and ranavirus presented with multifocal superficial necrotizing dermatitis.10 The reported histopathologic changes in the skin of the affected bearded dragon were similar to those reported in this case.9 Recurrent granulomatous dermatitis associated with PCR confirmed A. chelonae (syn. D. chelonae), was described in a king cobra (Ophiophagus hannah) in 2004.6,11 In 2019, a study of the endangered Chinese crocodile lizard (Shinisaurus crocodilurus) revealed cutaneous lesions associated with A. chelonae.4 Interestingly, experimental inoculation of the bacteria resulted in austwickiosis not only in Chinese skinks (Plestiodon chinensis), but also in domestic sheep, rabbits, and guinea pigs.4 This suggests that A. chelonae has the potential to infect a wider range of species, including mammals, though natural infections have not been reported.
Infections by other members of the family Dermatophilaceae have been reported in reptiles, mammals, birds, and humans.4 In crocodiles, reports of ventrally located brown skin lesions, colloquially called “brown spot disease”, has been reported in young captive crocodiles (Crocodylus porosus and C. novaeguineae) in Papua New Guinea, farmed saltwater crocodiles (C. porosus) in Australia, and farmed American alligators (Alligator mississippiensis) in the southeastern United States.2,3 Of the saltwater crocodiles evaluated in Australia, approximately 30% of animals with clinical dermatitis contained intralesional filamentous, Dermatophilus-like bacteria.2,3 In lizards, dermatophilosis has been reported in captive bearded dragons confirmed as D. congolensis on culture. Dermatophilus-like organisms have been observed by light microscopy in a number of other species, including bush anoles (Polychrus marmoratus), collared lizards (Crotaphytus collaris), green iguanas (Iguana iguana), Senegal chameleons (Chamaeleo senegalensis), savannah monitors (Varanus exanthematicus), and panther chameleons (Furcifer pardalis).3
Contributing Institution:
University of Florida
College of Veterinary Medicine
Department of Comparative, Diagnostic, and Population Medicine
Gainesville, Florida
https://cdpm.vetmed.ufl.edu/
JPC Diagnosis:
Skin: Dermatitis, necrotizing, multifocal to coalescing, with hyperkeratosis and numerous filamentous bacilli.
JPC Comment:
The contributor provides an excellent, thorough summary of austwickiosis in reptiles and
notes an unusual feature of this organism: the presence of a toxin, similar to diptheria toxin, previously unknown outside of the Corynebacterium genus.
Diptheria toxin (DT), produced by specific species of Corynebacterium, is the main virulence factor responsible for the respiratory, neurologic, and cardiopulmonary symptoms that characterize diptheria in humans.12 DT is encoded on a bacteriophage which is integrated into the bacterial genome at specific sites. Regulation of DT expression is reliant on bacterial chromosome encoded diptheria toxin repressor genes, whose products can bind bacterial DNA sequences and block transcription and expression of DT.12
DT is considered the first example of the A-B class of toxins, an evolutionarily conserved motif found in almost all intracellular toxins.12 DT is produced as an inactive proenzyme that is cleaved to its active form on encountering a target cell. The two resulting peptides are DT-A, the catalytic portion of the toxin, and DT-B, which is required for receptor binding and subsequent translocation of the toxin into the target cell by receptor-mediated endocytosis.12
Once inside the cell, the acidic environment of the endosome causes a conformational change in DT, which causes the DT-A catalytic portion of the toxin to insert into the endosome membrane, exposing DT-A to the cytosol.12 DT-A catalyzes the transfer of an ADP compound from NAD+ to a histidine residue on elongation factor-2 (EF-2). EF-2 catalyzes the movement of ribosomes along mRNA during translation, making it essential for protein synthesis. When EF-2 is modified by the action of DT-A, the ribosome is unable to move, translation is halted, and the cell ultimately dies.12 DT-A disables EF-2 in all eukaryotic species except mice and rats, and one DT-A molecule exposed to the cytosol is enough to kill a cell.12
Diptheria-toxin like genes were identified in 2018 in several bacteria outside of the Corynebacterium genus, including Austwickia and Streptomyces spp.13 The key features of the toxin, including the catalytic and translocation domains, are conserved, but the receptor-binding domains are different, accounting for the different host ranges and cellular tropisms of the DT-like toxin in non-Corynebacterium organisms.12 In addition, DT-like toxin is encoded in the bacterial genome of the new hosts rather than on bacteriophages, suggesting an alternate method of lateral gene transfer has occurred in these species.12
It is unclear if DT-like toxin has a role in the pathogenesis of A. chelonae infection; however, abundant necrosis is a hallmark of this disease in all species, raising the possibility that DT-like toxin causes the same cellular injury via the same mechanism as its DT ancestor.
Discussion of this case initially centered on the abundant autolysis which caused interpretive challenges for conference participants. Participants noted that the abundant filamentous bacteria were seemingly not accompanied by the a robust inflammatory reaction, prompting a lengthy discussion about whether the bacteria could represent post-mortem bacterial overgrowth rather than true pathogenic bacteria. On closer inspection, however, participant believed that some of the ghost cells in the tissue are presumptive heterophils that have been rendered inapparent due to autolysis.
The organism itself cuts a striking filamentous figure in this slide. The moderator noted that this morphologic feature can be used to narrow the differential list considerably to the most commonly-encountered filamentous bacteria: Actinomyces, Nocardia, Dermatophilus, and Streptobacillus. The moderator also reminded participants not to neglect the bone marrow The bone marrow in this case appears mildly hypocellular, which comports with the observed mild inflammation. Dr. Terio noted that ambient temperature has a profound impact on the ability of poikilotherms to mount inflammatory responses and speculated that low body temperature might explain the disconnect between the level of inflammation and the extent of bacterial infection observed in this animal.
References:
- Bemis DA, Patton CS, Ramsay EC. Dermatophilosis in captive tortoises. J Vet Diagn Invest. 1999;11(6):553-7.
- Buenviaje GN, Ladds PW, Martin Y. Pathology of skin diseases in crocodiles. Aust Vet J. 1998;76(5):357-63.
- Jacobson ER, Garner MM. Actinomycetales- Dermatophilus and Austwickia. In: Infectious diseases and pathology of reptiles-color atlas and text. 2nd ed. CRC Press; 2020.
- Jiang H, Zhang X, Li L, et al. Identification of Austwickia chelonae as cause of cutaneous granuloma in endangered crocodile lizards using metataxonomics. PeerJ. 2019;7:e6574
- Kagia K, Lieu WT. The Family Dermatophilaceae. In: Roseberg E, The Prokaryotes-Actinobacteria. 4th ed. Springer-Verlag Berlin Heidelberg; 2014
- Latney LV, Wellehan JFX. Selected Emerging Infectious Diseases of Squamata: An Update. Vet Clin North Am Exot Anim Pract. 2020;23(2):353-371.
- Liguori BL, Ossiboff RJ, Stacy NI, et al. Austwickia chelonae in a wild gopher tortoise (Gopherus Polyphemus) and evidence of positive selection on the diphtheria-like toxin gene. J Wildl Dis. 2022;58 (1):1-7.
- Masters AM, Ellis TM, Carson JM, Sutherland SS, Gregory AR. Dermatophilus chelonae sp. nov., isolated from chelonids in Australia. Int J Syst Bacteriol. 1995;45: 50-56
- Rostad SJ, Brandão J, Ramachandran A, Chien RC, Confer AW. Austwickiosis in captive African Spurred Tortoises (Geochelone sulcata) co-infected with Cryptosporidium ducismarci. J Comp Pathol. 2019;173:1-7.
- Tamukai K, Tokiwa T, Kobayashi H, Une Y. Ranavirus in an outbreak of dermatophilosis in captive inland bearded dragons (Pogona vitticeps). Vet Dermatol. 2016;27(2):99-105e28.
- Wellehan JF, Turenne C, Heard DJ, Detrisac CJ, O'Kelley JJ. Dermatophilus chelonae in a king cobra (Ophiophagus hannah). J Zoo Wildl Med. 2004;35(4):553-6.
- Young LS. New Corynebacterium species with the potential to produce diptheria toxin. Pathogens. 2022;11(11):1264.
- Mansfield MJ, Sugiman-Marangos SN, Melnyk RA, Doxey AC. Identification of a diptheria toxin-like gene family beyond the Corynebacterium genus. FEBS Lett. 2018;592(16):2693-2705.