Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 10
20 November 2019
CASE IV: 19-33870
(JPC 4138045).
Signalment: Adult, female Silvery Salamander (Ambystoma platineum)
History: This wild salamander was found dead in the water in late March at a local state park in central Illinois.
Gross Pathology: Examined is a 12.0 g adult female Silvery Salamander (Ambystoma platineum) in fair postmortem condition. There are numerous, discrete, 1-3 mm in diameter white, flat to slightly raised cutaneous foci scattered throughout the entire surface of the body (Figure 1). There are few discrete 1-2 mm in diameter white to tan flat foci within the subcapsular surface of the liver. The remaining viscera are unremarkable.
Laboratory results:
FV3 Ranavirus qPCR was negative.
Microscopic Description:
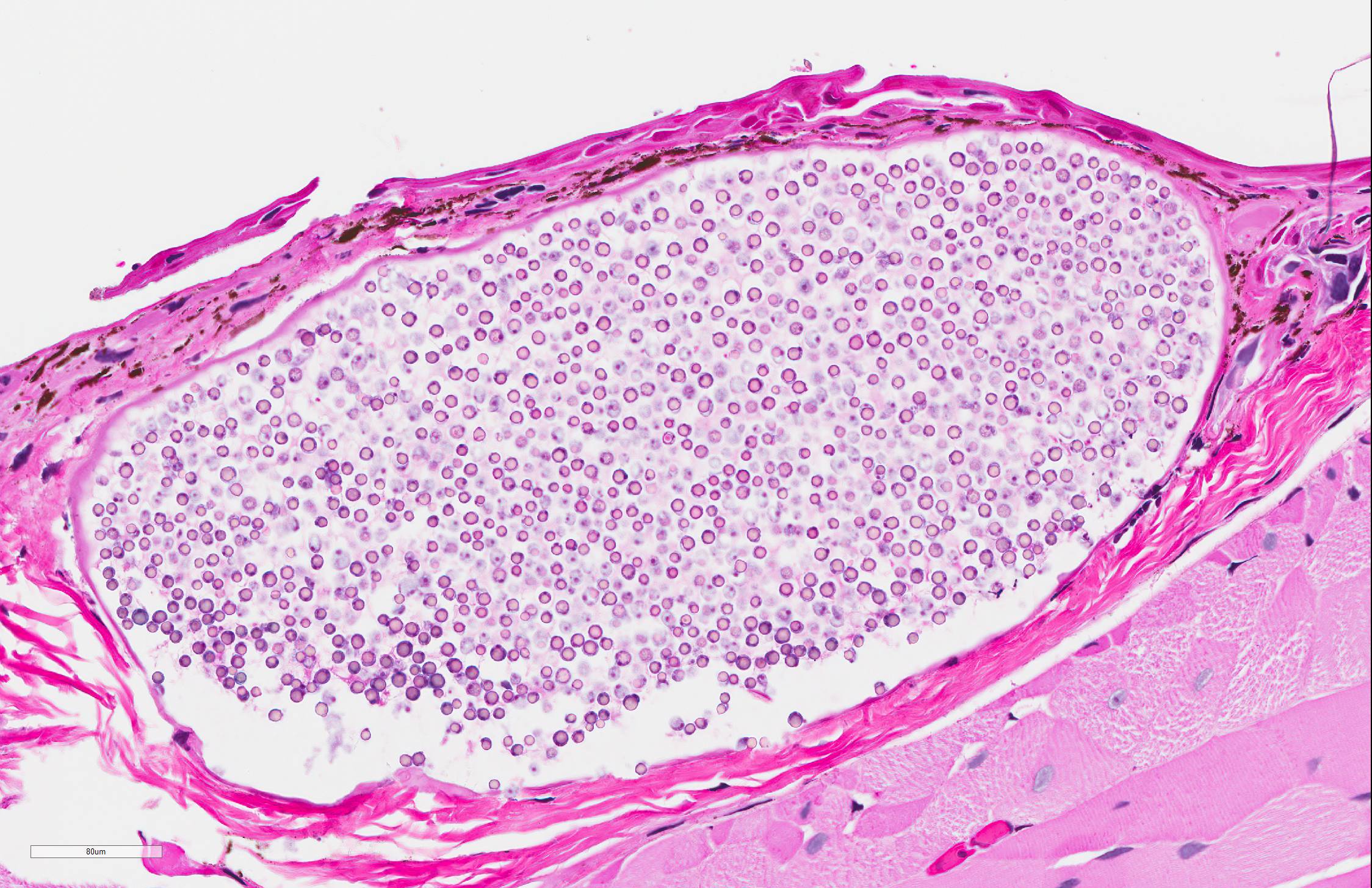
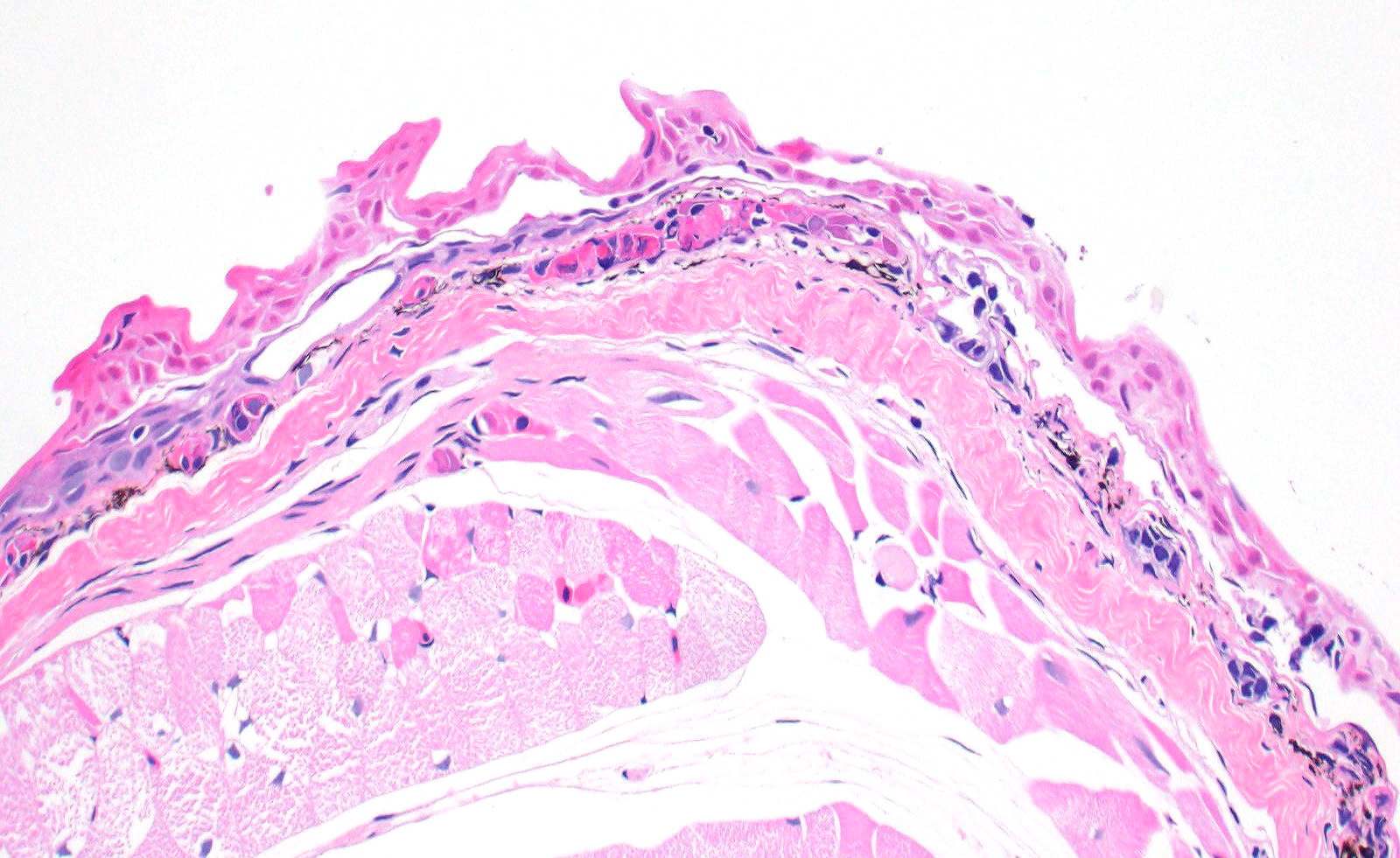
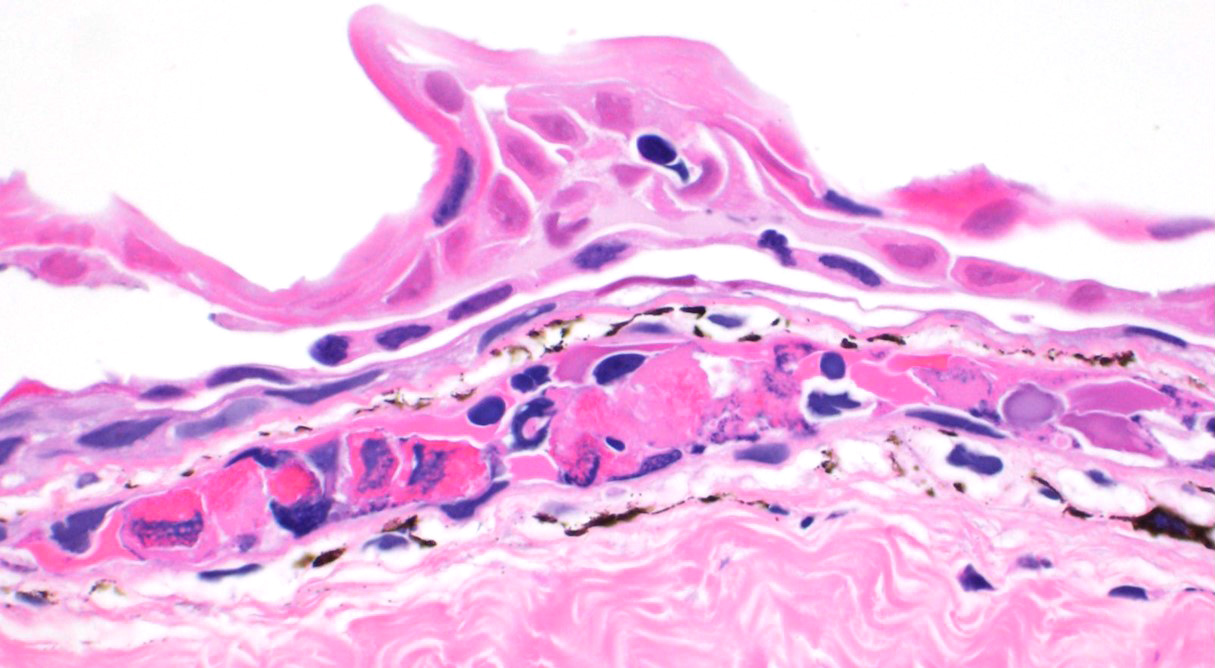
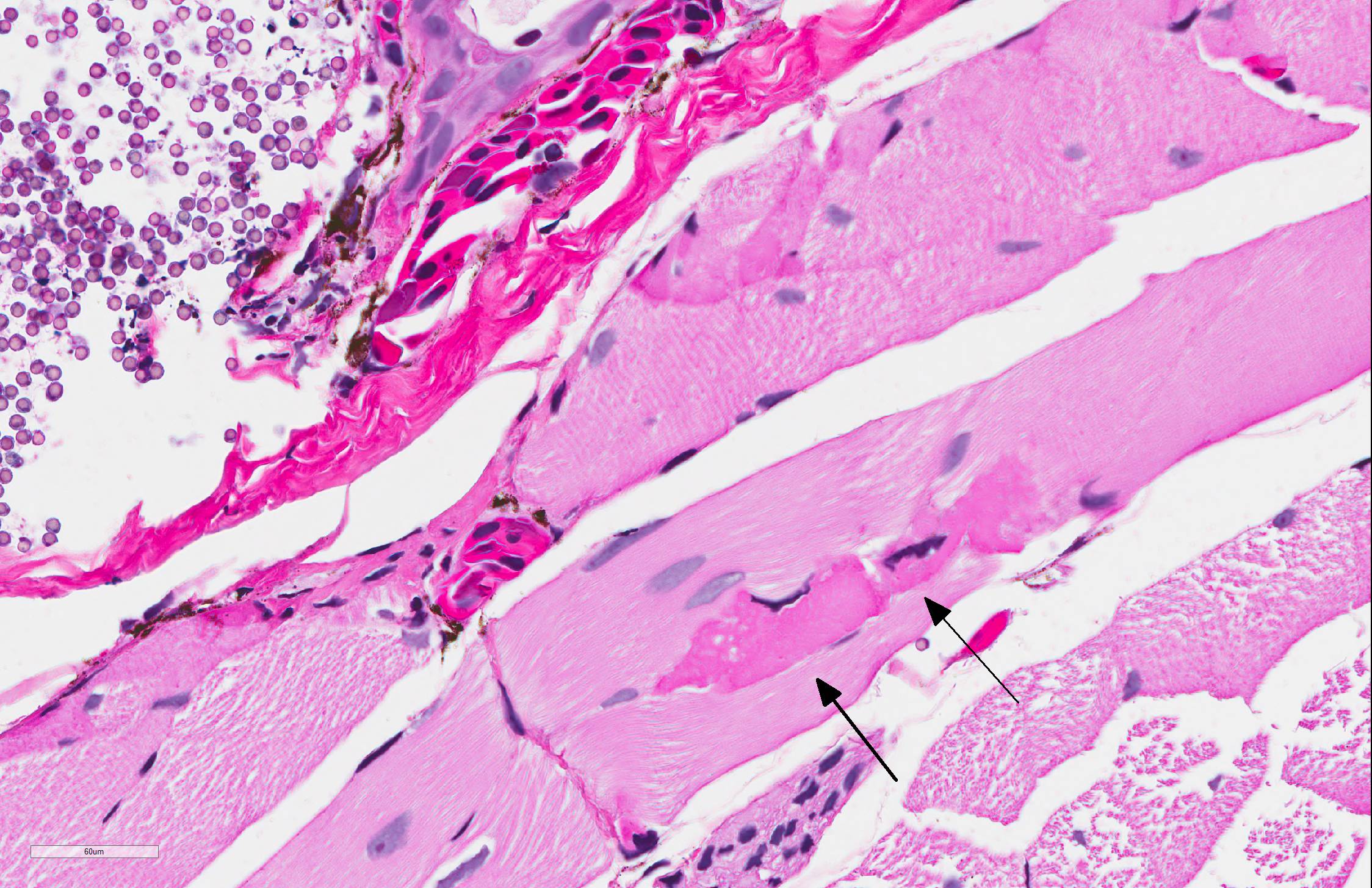
Skin: Mostly confined within the epidermis and rarely expanding the dermis are large numbers of variably sized intraepidermal cysts (sporangia) packed with round, 5-10 µmin diameter, eosinophilic globular spores with scant crescent shaped pale eosinophilic variably granulated cytoplasm. The sporangia mostly distend the mid to basal portions of the epidermis. There are moderate numbers of coccobacilli enmeshed in between the spores. There is minimal leukocytic reaction towards the cysts. The remaining epidermis is moderately hyperplastic with multiple segments of prominent superficial cornification.
Special staining of sporangia within the liver with Grocott's Methenamine Silver (GMS) and Periodic acid-Schiff (PAS) is performed. The GMS stain highlights in black the majority of the spore's capsule and parts of the internal contents.
Diffusely, the entire spore structure has strong reaction for PAS.
Other findings: (Not included in slide submission)
Intestine: There are moderate numbers of apicomplexan organisms in various stages of development within the intestinal mucosa. Mucosal enterocytes show intraepithelial microgametes and macrogametes. Some enterocytes appear degenerate and vacuolated with karyomegalic intranuclear basophilic inclusions. The lamina propria is infiltrated by a small number of lymphocytes, plasma cells, and heterophils.
Lungs: Occasional, pneumocytes have karyomegalic nuclei with intranuclear, basophilic viral inclusions.
Pancreas: Pancreatic acinar cells occasionally have karyomegalic nuclei with intranuclear, basophilic viral inclusions.
Kidney: Renal tubular epithelium occasionally have karyomegalic nuclei with intranuclear, basophilic viral inclusions.
Contributor Morphologic Diagnosis:
Skin (bodywide): Numerous intraepidermal Mesomycetozoan sporangia
Contributor Etiologic diagnosis: Cutaneous amphiocystidiosis
Contributor Comment: The cause of death in this salamander is likely multifactorial. The presence of cutaneous amphiodiocystidiosis may be considered an incidental finding, however the concurrent visceral involvement (liver) in this case might suggest significant infection secondary to immune suppression or damaged defense mechanisms. A primary infectious etiology was not identified, so clinical deterioration due to environmental factors cannot be ruled out.
Amphibiocystidium is a genus of fungal-like parasites that cause characteristic skin nodules in amphibians of Europe and North and South America. A member of the class Mesomycetozoea, these protists arose at the animal-fungal divergence and were historically considered either protozoan or fungal.7
Mesomycetozoans are found in marine and freshwater environments as commensals or parasites to invertebrates, fish, amphibians, birds, and mammals.8 Amphibiocystidium is closely related to the genuses Dermocystidium, which is a primary pathogen of salmonid fish, and Rhinosporidium, which causes clinical disease in birds and mammals, including humans.7
Grossly, Amphibiocystidium spp. cause multifocal, regular, 3-5 mm in diameter often spherical, "C" or "U"-shaped skin nodules that are sometimes ulcerated. These lesions must be grossly differentiated from encysted parasites (particularly Clinostomum sp. trematodes) or granulomas caused by higher order bacteria or fungi.
Microscopically, studies report several 100-600 µm cysts (sporangia) containing myriad 2-6 µm endospores.7 An inflammatory cell infiltrate composed primarily of lymphocytes and macrophages may be found in the vicinity of these cysts. The associated epidermis may be hyperplastic. Histologic lesions must be differentiated from unidentified alveolate protozoa observed in ranid frogs, which have been associated with mortality events in free-ranging ranid tadpoles in the United States.8 Tissues affected by alveolate protozoa will be infiltrated by large numbers of 6-9 µm spherical basophilic spores in absence of a host inflammatory response, and spores are positive for periodic acid-Schiff (PAS), Grocott's methenamine silver (GMS), and Congo Red stains.8
The phylogeny of Amphibiocystidium
is still unsettled; the genus was recently created to encompass members of
the Dermocystidium, Dermomycoides, and Dermosporidium spp.
affecting amphibians, interpreted as monophyletic,6 and may also
include the related genus Amphibiothecum, a parasite of American toads (Anaxyrus
americanus). Predominant species of Amphibiocystidium include A. ranae, A. viridescens. and
A. pusula.7
Amphibiocystidium spp. have been reported to affect both anuran and caudate species in Europe, where it has been detected for over a century, and was more recently discovered in North America.9 Although lethal infections have been documented, the large majority of reported cases are associated with low morbidity and mortality.2 The life cycle and pathogenesis of Amphibiocystidium is largely unknown.3 Although clinical challenge studies have yet to be performed, a 7-year survey of a natural population of Italian stream frogs (Rana italica) found no appreciable negative effects of Amphibiocystidium infection; however, ecological and host-specific factors influencing susceptibility were not assessed in this study.2
Primarily attributed to climate change and emerging pathogens such as chytrid fungus (Batrachochytrium dendrobatidis) and ranaviruses, the current massive decline in global amphibian populations has emphasized the need to understand the myriad of other infectious agents affecting amphibian species, most of which have yet to be well-described and understood.9,10 Some of these agents, such as Amphibiocystidium, have been found in amphibians co-infected with Batrachochytrium dendrobatidis, with several other reports implying but not confirming co-infection.1 Many of these agents, such as Amphibiocystidium have unknown pathogeneses and have yet to undergo clinical studies.
Contributing Institution:
University of
Illinois at Urbana-Champaign, Veterinary Diagnostic Laboratory
http://vetmed.illinois.edu/vet-resources/veterinary-diagnostic-laboratory/
JPC Diagnosis: 1. Skin, epidermis: Numerous sporangia.
2. Skin, epidermis: Necrosis, multifocal, with vasculitis, dermatitis, and epidermal
hyperplasia.
JPC Comment: The contributor
has done an outstanding job in this review of Amphibiocystidium, an
emerging mesomycetozoan pathogen.
The Mesomycetozoae are a class of organisms that contain ten different genera of
saprophytic and parasitic organisms (grouped by 18s ribosomal DNA analysis)
which have changed in classification over the years, previously being referred
to as both fungi and protists. Their grouping was initially called the âDRIP
cladeâ, an acronym composed of its initial members Dermocystidium, rosette
agent, Ichthyophonus and Psorospermium. With the exception of Rhinosporidium,
parasitic species primarily infect aquatic animals. Most of these agents have
poorly documented life cycles.5
Rhinosporidium
seeberi
is the most well-known of the group, due to its propensity to infect higher
vertebrates, including dogs, horses, and humans. It is thought that most
infections arise via traumatized epithelium. Nasal polyps are the most common
and identifiable lesions associated with infection and in humans (account for
70% of lesions; however, humans have demonstrated a range of other staging
areas, including the palpebral and bulbar conjunctiva (15%), sclera, lacrimal
sac, and genital mucous membranes. No immunity is developed against
rhinosporidiosis, although rarely spontaneous regression has been noted.4
The term "rosette agent" refers to a species of mesomycetozoan (Sphaerothecum
destruens) which was first identified in China and appears to have been
spread through the aquaculture trade to Europe and the US in association with
another invasive species, its natural host the stone moroko (Pseudorasbora
parva). It has been identified in over 4 species, including salmon, carp,
and sea bass. This mesomycetozoan results in systemic and often fatal infection
in aberrant hosts, but invading gills, liver, kidney, intestine, and gonads.
The spores pass from infected fish in both urine and seminal fluid. The
parasite has the ability to infect both salmonids and cyprinids, with great
potential to impact global piscine biodiversity as well as have a profound
impact on aquaculture and food security.12
Mesomycetozae
have made a number of appearances in the Wednesday Slide Conference:
Rhinosporidium seeberi: (WSC 2017-2018 Conf 3, Case 3-horse, WSC
2016-2017 Conf 8, Case 1-horse, WSC 2010-2011 Conf 14, Case 3-horse, WSC
2002-2003 Conf 11, Case 3-dog, among others); Ichthyophonus (WSC
2001-2002 Conf 24, Case 4-rainbow trout, WSC 2015-2016 Conf 13, Case 1-red-spotted
newt); Dermocystidium (WSC 2015-2016 Conf 13, Case 1-tetra, WSC
2010-2011 Conf 22, Case 3-koi); and Perkinsus (classified at the time on
the shifting sands of the mesomycetozoans-but now classified as in the phylum
Perkinsozoa) (WSC 2013-2014, Conf 12, Case 3-abalone).
A pathologist from the National Zoo in attendance commented on the presence of myonecrosis in this slide and the frequency with which it is seen in fresh autopsies on amphibians, to the point that he considered these changes to be almost artifactual in this case. Several participants, including the moderator saw areas in which the epidermis was diffusely pink and without differential staining, and mildly separated with serum pooling. One area was associated with a focal area of granulocyte aggregation. Spirited discussion greeted this finding (which may not have been present on all slides), but its cause is unclear. The moderator also discussed the peculiar life cycle of this gynogenic species, in which all specimens are female, and mating with males of another species. The sperm of the male (usually blue-spotted or Jefferson salamanders) stimulates egg development, but does not contribute to the DNA of the offspring. The moderator also discussed the difficulty (per Dr. Alan Pessier of Washington State University) of definitively differentiating Amphibiocystidium from Perkinsus based on HE slides.
References:
1. Borteiro C, Cruz JC, Kolenc F, et al. Dermocystid-chytrid coinfection in the Neotropical frog Hypsiboas pulchellus (Anura: Hylidae). J Wildl Dis. 2014;50(1):150-153.
2. Fagotti A, Rossi R, Canestrelli D, et al. Longitudinal study of Amphibiocystidium sp. infection in a natural population of the Italian stream frog (Rana italica). Parasitology. 2019:1-8.
3. Gonzalez-Hernandez M, Denoel M, Duffus AJ, Garner TW, Cunningham AA, Acevedo-Whitehouse K. Dermocystid infection and associated skin lesions in free living palmate newts (Lissotriton helveticus) from Southern France. Parasitology International. 2010;59(3):344-50.
4. John D, Selvin SST, Irodi A, Jacob P. Disseminated rhinosporidiosis with conjunctival involvement in an immunocompromised patient. Mid East Afr J Ophthal 2017; 24(1):51-53.
5.
Mendoza
L, Taylor JW, Ajello L. The class Mesomycetozoea: a heterogenous group
of micro-organisms at the animal-fungal boundary. Ann Rev Microbiol
2002; 56:315-244.
Pascolini R, Daszak P, Cunningham AA, et al. Parasitism by Dermocystidium ranae
in a population of Rana esculenta complex in Central Italy and description
of Amphibiocystidium n. gen. Dis Aquat Org.
2003;56(1):65-74.
6. Pereira CN, Di Rosa I, Fagotti A, Simoncelli F, Pascolini R, Mendoza L. The pathogen of frogs Amphibiocystidium ranae is a member of the order Dermocystida in the class Mesomycetozoea. J Clin Microbial. 2005;43(1 ):192-198
7. Pessier A. Amphibia. In: Terio, K. A., McAloose, D., & Leger, J. S, eds. Pathology of Wildlife and Zoo Animals. Academic Press; 2018:915-944.
8. Pessier AP. Hopping over red leg: The metamorphosis of amphibian pathology. Vet Path. 2017;54(3):355-357
9. Pessier AP. Management of disease as a threat to amphibian conservation. International Zoo Yearbook. 2008;42(1):30-39.
10. Raffel TR, Bommarito T, Barry DS, Witiak SM, Shackelton LA. Widespread infection of the Eastern red-spotted newt (Notophthalmus viridescens) by a new species of Amphibiocystidium, a genus of fungus-like mesomycetozoan parasites not previously reported in North America. Parasitology. 2008;135(2):203-215.
11.
Sana
S, Harodouin EA, Gozlan RE, Ercan D, Tarkan AS, Zhang T, Andreou D. Origin and
invasion f the emerging infectious pathogen Sphaerothecum destruens.