Signalment:
Gross Description:
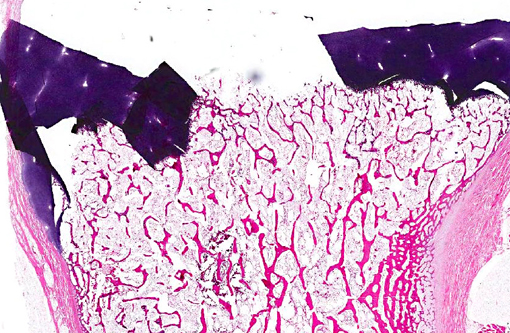
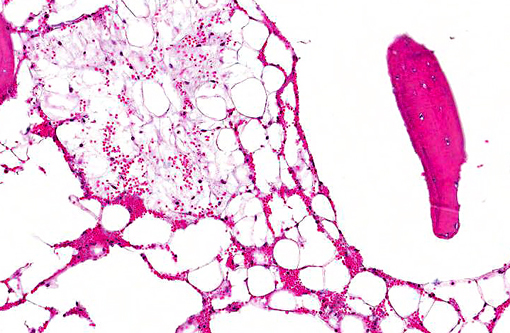
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Aplastic pancytopenia has historically been uncommon in cattle but has been documented in association with infection with BVDV type 2.(11) BVDV type 1 has been isolated from cases of fatal hemorrhagic thrombocytopenia but experimental infection has failed to replicate the condition.(7) Fatal hemorrhagic pancytopenia has also been reported in cattle due to ingestion of bracken fern(10) and of T-2 mycotoxins.(8)
JPC Diagnosis:
Conference Comment:
Alloimmune phenomena are also described in several other species, including foals, humans and piglets. Neonatal isoerythrolysis (NI) in foals, a common alloimmune disease in foals, is a type II hypersensitivity reaction caused by the presence of anti-erythrocyte antibody complexes in the colostrum of the dam and results in destruction of erythrocytes.(3) This condition occurs in horses because of exposure to an incompatible blood type from the stallion. NI has also been reported in cattle and cats.(6) In neonatal alloimmune thrombocytopenia (NAIT), women homozygous for a certain genetic trait, who are carrying a fetus with heterozygous platelet antigens partially inherited from the father, may develop anti Human Platelet Antigen 1 antibodies. These antibodies pass through the placenta, causing fetal thrombocytopenia and subsequent intracranial hemorrhage.(1) NAIT has also been described in foals and piglets. In foals, it is hypothesized that the mares plasma and milk contains antibodies reactive to foal platelet antigens inherited from the sire.(5) In contrast to BNP, which affects all hematopoietic cell lines, these conditions target one cell line; NI impacts erythrocytes while NAIT targets platelets, resulting in hemolytic anemia and thrombocytopenia respectively.(1,5)
In general, viruses and toxins are the most common causes of pancytopenia. In addition to those infectious/toxic agents described by the contributor, further potential causes of pancytopenia include radiation, chemotherapeutic agents, estrogen toxicity, stachybotryotoxicosis, infectious agents and myelophthisis.(10,11) Chemotherapy induced myelosuppression is the most common cause of canine pancytopenia, and is typically associated with doxorubicin administration.(11) Estrogen compounds, which may originate from iatrogenic administration or hormone overproduction, are also myelotoxic. Disease is typically characterized by irreversible pancytopenia with widespread hemorrhage.(10) Stachybotryotoxicosis is a pancytopenic disease of horses and ruminants that occurs due to ingestion of feed contaminated with the fungus Stachybotrys alternates.(10) Feline and canine parvoviruses target proliferating cells, such as epithelial cells within intestinal crypts or hematopoietic cells within the bone marrow, resulting in pancytopenia.(10,12) Myelophthisis refers to replacement of normal hematopoietic bone marrow elements with abnormal tissue, usually neoplastic cells or fibrous connective tissue.(6) Bone marrow neoplasia can be primary (multiple myeloma, leukemia, lymphoma) or metastatic.(6) Malignant histiocytosis has also been associated with canine pancytopenia, likely due to a combination of decreased marrow production and cytophagia.(12) Bone marrow fibrosis, or myelofibrosis, is most commonly reported in dogs, and may occur with sepsis, neoplasia, drug toxicity and immune-mediated disease.(6)
References:
2. Bell CR, Scott PR, Sargison ND, Wilson DJ, Morrison L, Howie F, et al. Idiopathic bovine neonatal pancytopenia in a Scottish beef herd. Vet Rec. 2010;167:938-940.
3. Boyle AG, Magdesian KG, Ruby RE. Neonatal isoerythrolysis in horse foals and a mule foal: 18 cases (1988-2003). J Am Vet Med Assoc. 2005;227(8):1276-1283.
4. Bridger PS, Bauerfeind R, Wenzel L, Bauer N, Menge C, Thiel H-J, et al. Detection of colostrum-derived alloantibodies in calves with bovine neonatal pancytopenia. Vet Immunol Immunopathol. 2011;141:1-10.
5. Buechner-Maxwell V, Scott MA, Godber L, Kristensen A. Neonatal alloimmune thrombocytopenia in a Quarter horse foal. J Vet Int Med. 1997;11(5):304-308.
6. Fry MM, McGavin MD. Bone marrow, blood cells, and the lymphatic system. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:704-734.
7. Hamers C, Couvreur B, Dehan P, Letellier C, Lewalle P, Pastoret PP, et al. Differences in experimental virulence of bovine viral diarrhoea viral strains isolated from haemorrhagic syndromes. Vet J.2000;160:250-258.
8. Hsu IC, Smalley EB, Strong FM, Ribelin WE. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl Microbiol. 1972;24:684-690.
9. Pardon B, Steukers L, Dierick J, Ducatelle R, Saey V, Maes S, et al. Haemorrhagic diathesis in neonatal calves: an emerging syndrome in Europe. Transbound Emerg Dis. 2010;7:135146.
10. Valli VEO. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 3. Philadelphia, PA: Elsevier; 2007:216-220.Â
11. Walz PH, Bell TG, Steficek BA, Kaiser L, Maes RK, Baker JC. Experimental model of type II bovine viral diarrhea virus-induced thrombocytopenia in neonatal calves. J Vet Diagn Invest. 1999;11:505-514.Â
12. Weiss DJ, Evanson OA, Sykes J. A retrospective study of canine pancytopenia. Vet Clin Pathol. 1999;28(3):83-88.