Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 13
2 January, 2018
CASE IV: 317048 (JPC 4033739).
Signalment: 2-year and 6-month old female neutered domestic shorthair cat (Felis catus)
History: The animal was presented to the Internal Medicine department of the University Small Animal Hospital in the University of Glasgow School of Veterinary Medicine. The animal had a 6 week history of pica (licking coal) with a more recent history of reduced appetite and lethargy culminating to progressive deterioration of body condition. The animal was again referred to the Small Animal Hospital of the University and found to be tachypnoeic and lethargic. An echocardiogram showed thickening of the left ventricular wall and interventricular septum, mild pericardia! effusion and a moderate pleural effusion. 45ml of blood tinged fluid was drained from the thorax. The fluid had a high protein count with clotting protein noted within the syringes. The respiratory rate improved but shortly after respiratory and cardiac arrest ensued. The owners decided for the resuscitation procedure to be interrupted.
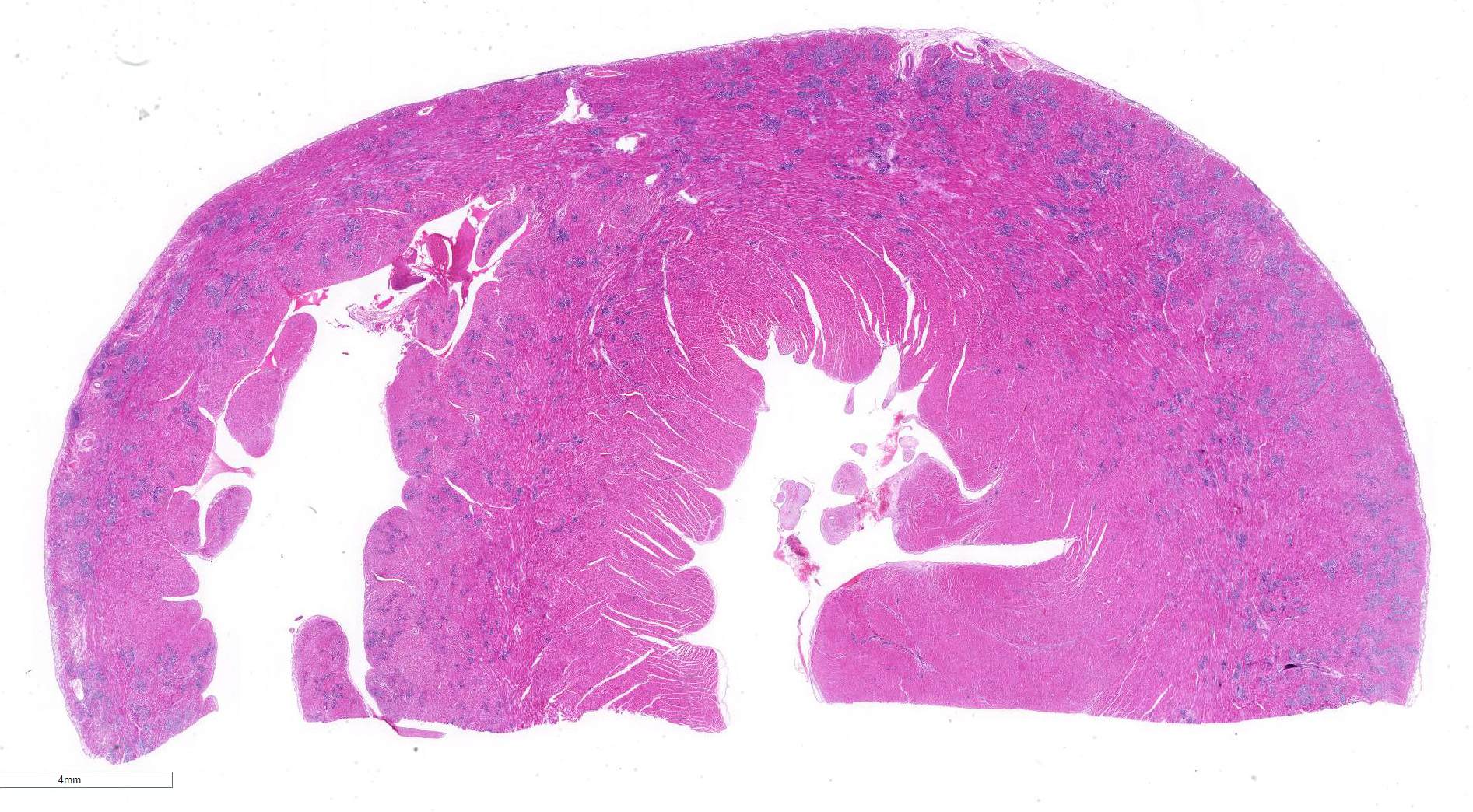
Gross Pathology: The animal was in fairly good general condition with considerable visceral deposits of adipose tissue. Within the pleural cavity there were approximately 50 ml of reddish fluid. Moderate amount of pinkish fluid is present within the trachea and extend to the bifurcation and bronchi. Multifocal areas of collapse are noted affecting multiple pulmonary lobes. Within the pericardial cavity there was a small amount (less than 10 ml) of reddish fluid. Thin fibrin tags were noted on the surface of the left atrium, and few 2x3 mm irregular areas of dark red discoloration were present on the epicardium in the upper portion of the left ventricle. The heart appeared moderately elongated and enlarged, and weighed 20.44 gr. The heart was fixed whole and re-evaluated a few days after fixation. Multiple transverse sections in the mid to lower portion of the ventricles were taken. The left ventricular wall and interventricular septum appeared moderately thickened with substantial reduction of the left ventricle lumen.
The spleen was moderately enlarged. The femoral bone marrow was diffusely plum red in color. In the urinary bladder there were multifocal ectatic venous vessels visible from the mucosal surface.
Laboratory results: Hematology evaluation showed a haematocrit of 20.8%, mild leukocytosis with a normal neutrophil count and thrombocytopaenia. A blood smear examination revealed moderate regeneration with polychromasia, anisocytosis and circulating red blood cell precursors. Testing for Mycoplasma haemofelis on a sample of peripheral blood was negative. There were some platelet aggregates on examination and on visual examination her platelet count was between 45-60x10A9/I.
Clinical chemistry revealed a mild azotemia, increases in ALT (290U/I) and AST
(219U/I) and a mild increase in total bilirubin.
Slide agglutination test was unremarkable and Coombs test was negative.
Microscopic Description:
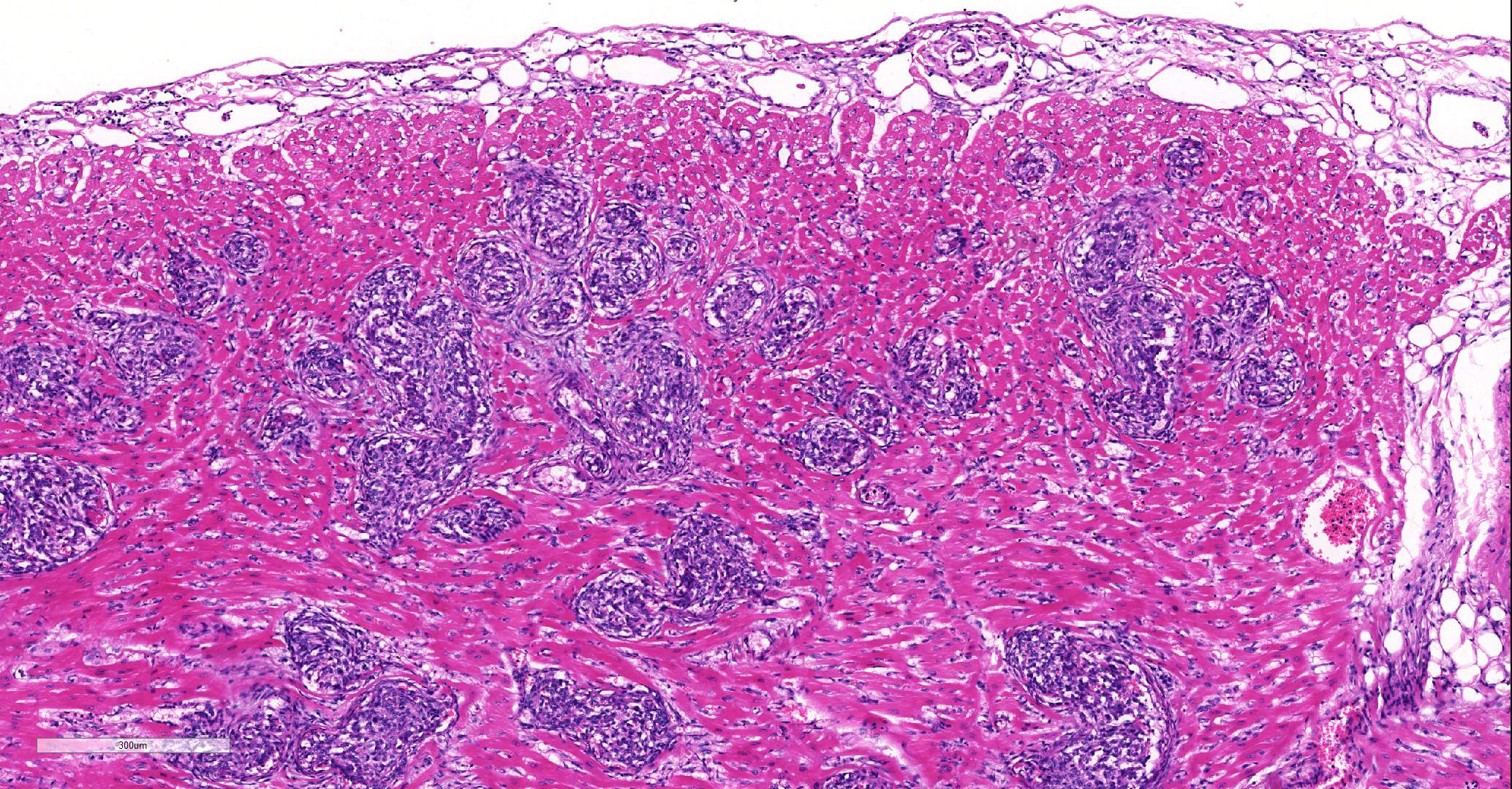
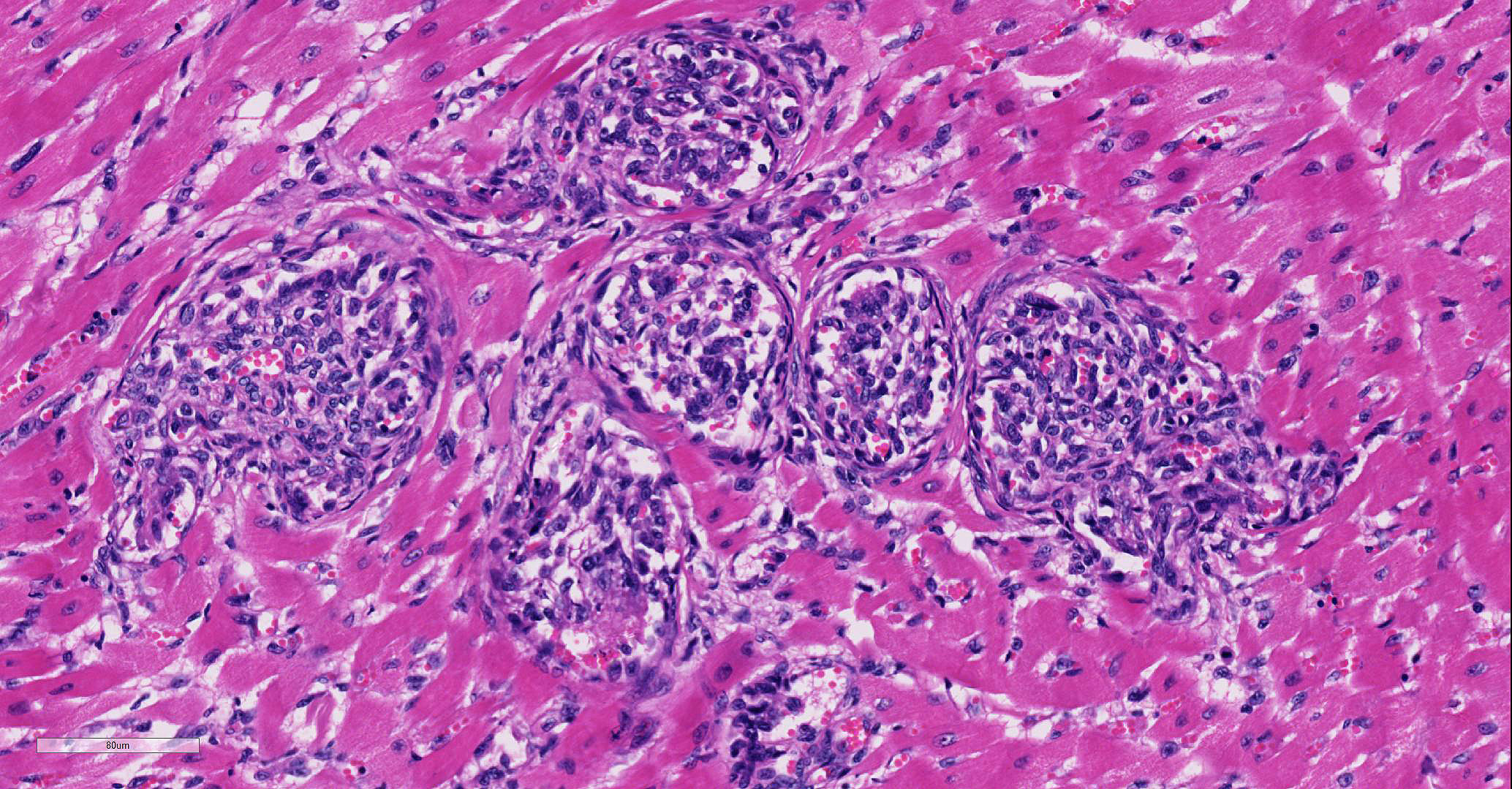
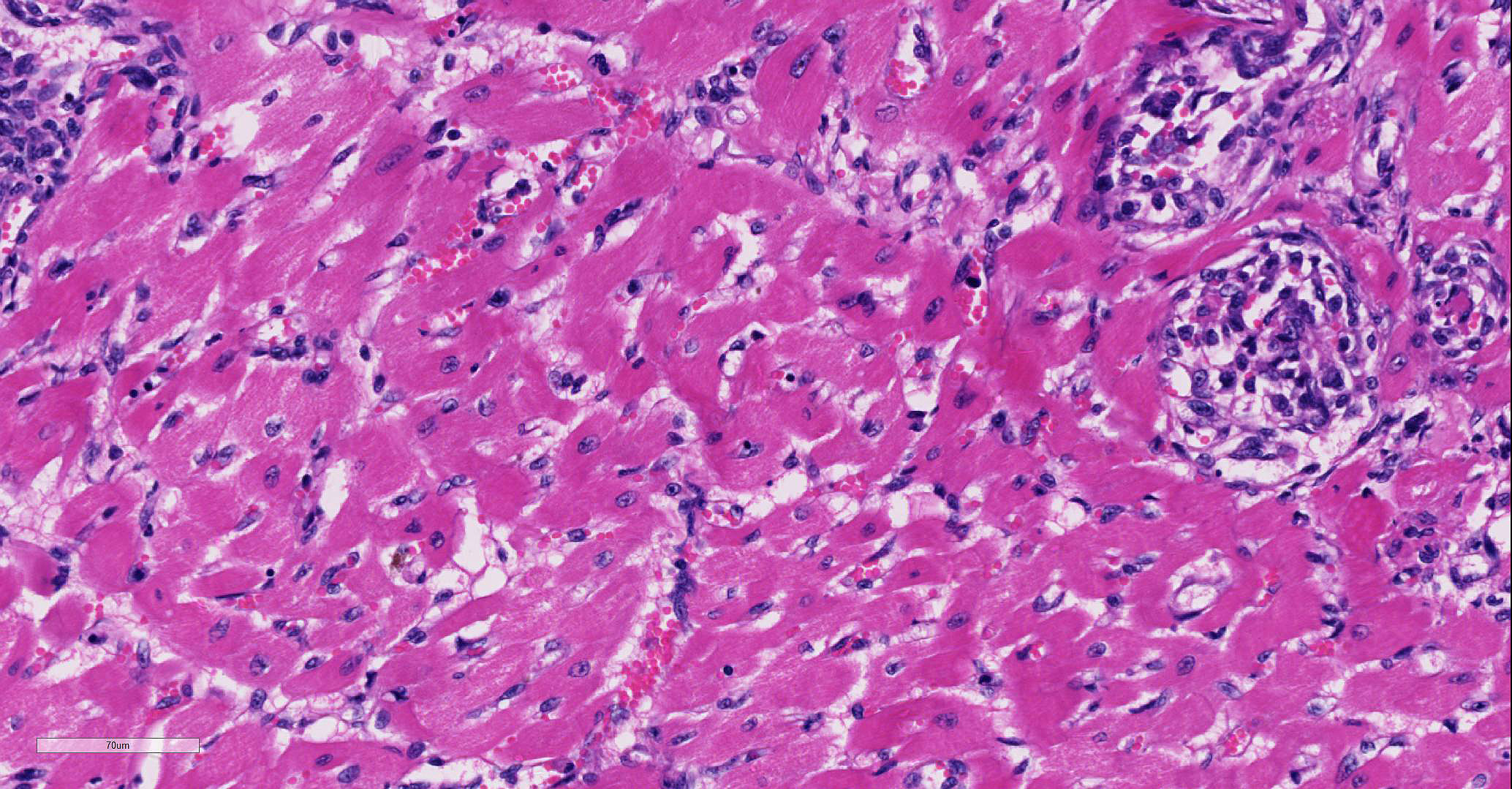
Involving approximately 30-40% of the tissue within the myocardium of the left and right ventricle and interventricular septum, there are proliferative changes multifocally affecting small to medium sized vessels. The vascular changes are characterised by proliferation of haphazardly arranged spindle cells frequently delimiting small slit-like spaces frequently showing the presence of erythrocytes and occasional fibrin thrombi. Some proliferations, characterised by a round shape and irregularly anastomosed vascular spaces, are reminiscent of glomeruloid structures. Multifocal variably sized areas of oedema with separation and disruption of cardiomyocytes are detectable, in some instances characterised by accumulation of small numbers of extravasated erythrocytes (microhaemorrhages). Scattered foci or small areas of cardiomyocyte condensation, shrinkage and fragmentation (degeneration and necrosis) are detectable with some variation among the sections. Focal areas of myocardial and subendocardial haemorrhage affecting a portion of a papillary muscle of the left ventricle are noted (but not present in all sections). Numerous mall infiltrates of moderate numbers of lymphocytes, plasma cells and few macrophages are noted multifocally along the epicardium.
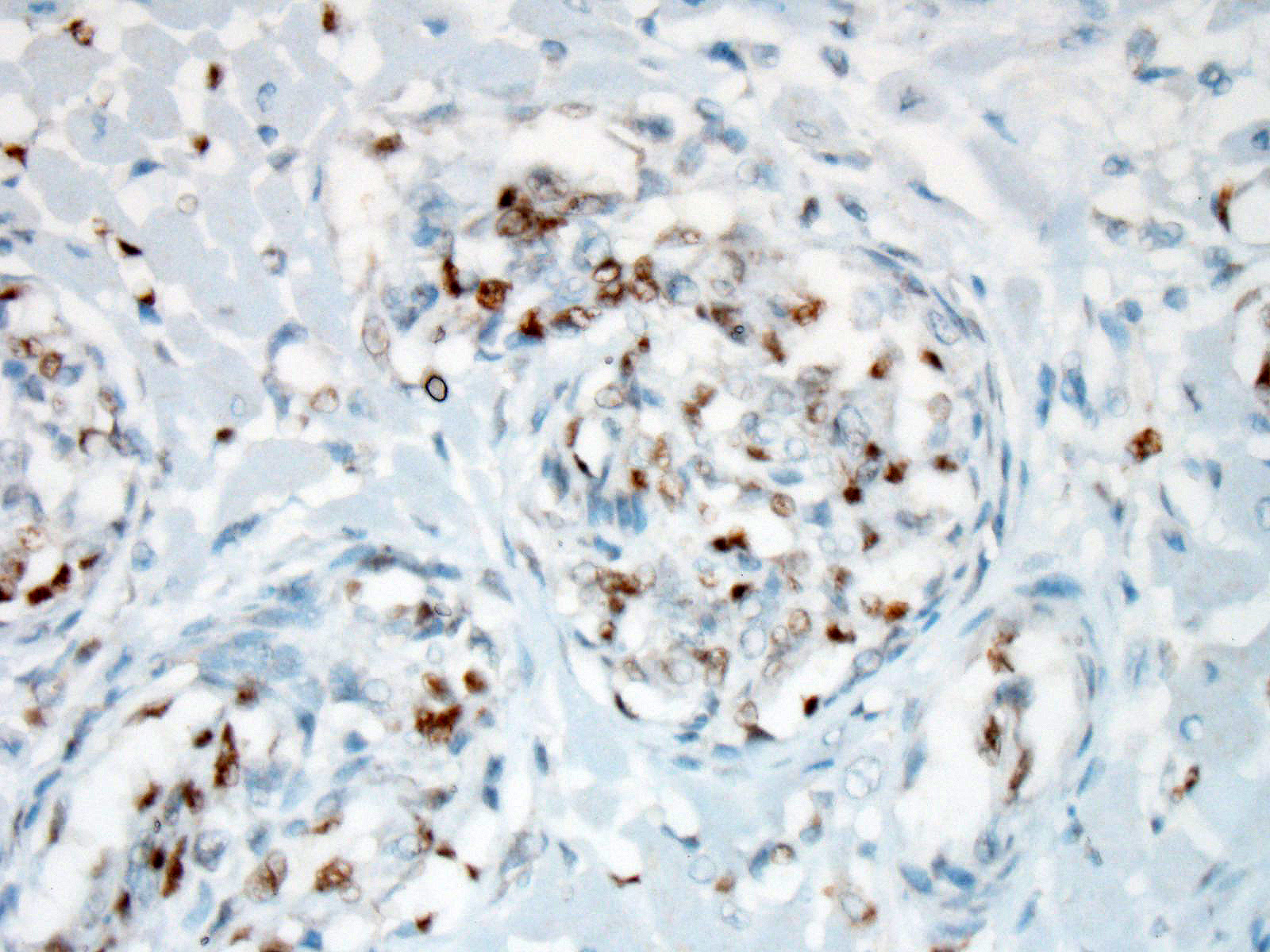
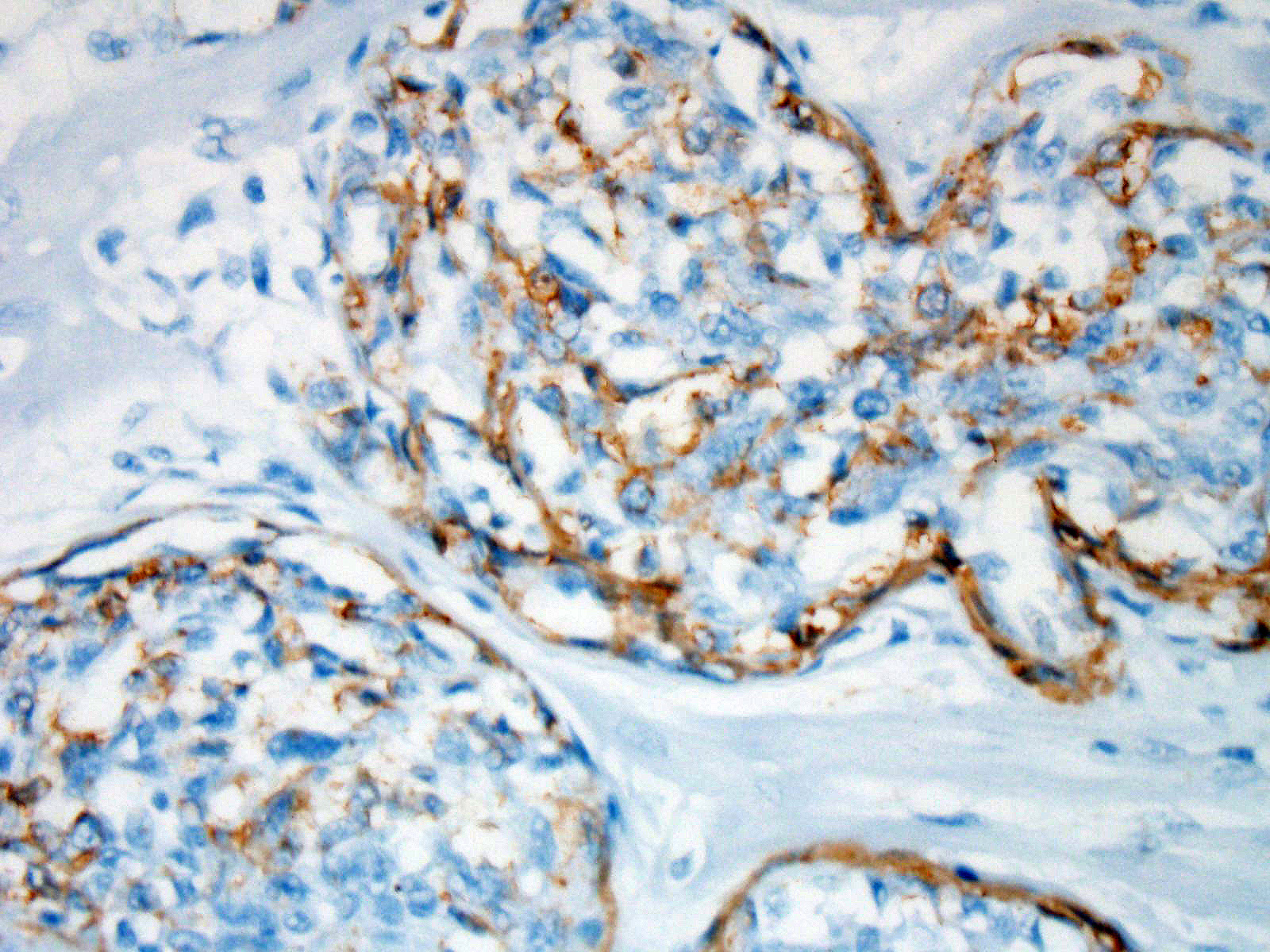
The plump spindloid cells comprising the vascular proliferative lesions were characterised by consistent moderate to strong cytoplasmic immunoreactivity for alpha smooth muscle actin (aSMA) and only occasional positive staining for van Willebrand Factor (vWF). On the other hand, strong vWF immunolabeling was consistently observed in fibrin and platelet thrombi associated with the small irregular and slit-like lumens of the vascular proliferative lesions.
Vascular proliferative changes similar to those observed in the heart were also noted in the spleen, in the bone marrow, and in the kidney, affecting the afferent arterioles at the vascular pole of the glomeruli, and to variable extents the of glomerular tuft capillaries.
Contributor’s Morphologic Diagnoses:
Heart, intravascular endothelial and pericyte proliferation, marked, multifocal, consistent with reactive angioendotheliomatosis
Contributor’s Comment: The term "angioendotheliomatosis" in human medicine has been a subject of controversy and confusion, resolved with the recognition of two distinct conditions:
"malignant angioendotheliomatosis" form representing a type of aggressive intravascular lymphoma, and "reactive angioendotheliomatosis" (RAE) which consists of endothelial cells and peric ·.n10•e 15 proliferative changes mainly affecting small to medium sized vessels of the skin. Notably, two cases of intravascular disseminated angiosarcoma were also reported in humans and discussed as examples of "true neoplastic angioendotheliomatosis".8
Seventeen cases of "malignant angioendotheliomatosis" (intravascular lymphoma) were described in the dog, with consistent involvement of the central nervous system and variable involvement of other tissues,9 and a single case was described in the cat.6 Sporadic cases of feline multisystemic disease characterized by vascular endothelial and pericyte proliferative lesions have been reported, addressing comparisons with different vasoproliferative conditions in humans and other animal species.4,11,12
A subsequent report describing a case series of cats affected by similar multisystemic vascular proliferative lesions and addressing a review of the literature, defined the criteria for the classification of this condition as feline systemic reactive angioendotheliomatosis.5 This rare condition in cats proves invariably fatal and frequently characterized by cardiac dysfunction and/or failure, variably associated with respiratory distress, in some cases hematological alterations. Serosanguinous and/or fibrinous pericardial effusions were reported in some instances, similar to those observed in our case. Microscopic evidence of vascular proliferative changes was most consistently observed in the heart in all cases. Spleen, kidney and lymph nodes were also frequently involved. Other organs affected at lower frequency included pancreas, gastrointestinal tract, brain, meninges, eyes, adrenals, spinal cord, liver, subcutis, thyroids, sciatic nerve and bone marrow.4·5,11-12
The etiopathogenesis of reactive angioendotheliomatosis remains obscure in both humans and cats. The possible association of reactive angioendotheliomatosis with underlying infection, thrombotic thrombocytopenic purpura (TTP), autoimmune disorders, hypersensitivity, cryoglobulinemia, renal and liver failure, post liver transplantation, bone marrow transplantation-related graft versus host disease has been discussed in humans7,10,15
A recent report described the detection of DNA of different Bartonella species, including B. vinsonii subsp. berkhoffii, B. henselae, and B. koehlerae from tissues of cats affected by systemic reactive angioendotheliomatosis.1 The authors also provide evidence for B. vinsonii subsp. berkhoffii causing upregulation of hypoxia inducible factor-1 a (HIF-1 a) with resulting increased production of vascular endothelial growth factor (VEGF) in an in vitro cell culture system.1
Lesions with features strikingly similar to feline systemic reactive angioendotheliomatosis were reported in a 2-year old steer presumed to be persistently infected with bovine viral diarrhea virus (BVDV). BVDV antigen was detected in a proportion of the glomeruloid intravascular proliferations.3 The authors addressed a possible comparison between the multisystemic vascular proliferative changes and a TTP-like condition ensuing in the animal. The possibility of relationship between BVDV infection and the development of the intravascular proliferative lesions was also discussed although a causal connection could not be definitively demonstrated.3 Interestingly, tissue samples from this animal were also submitted for molecular analysis and B. henselae DNA was isolated from the vascular proliferative lesions affecting that steer.1
These results offer interesting perspectives on the postulated association between infection with Bartonella spp. and the development of vascular proliferative changes including bacillary angiomatosis,16 epithelioid haemangioendothelioma,2 and hemangiosarcoma.2
However, a later study demonstrated that, in the context of diagnostic histopathology laboratories, multiple potential sources of cross-contamination associated with tissue processing may result in Bartonella spp. DNA carryover and need to be carefully monitored and excluded to ensure the validity and significance of Bartonella spp. isolation from paraffin embedded tissues.13
Immunohistochemical labeling performed at the JPC showed that some of the proliferative cells within the arterioles were positive for smooth muscle actin, while a second population within the proliferative masses were simultaneous immunopositive for Factor VIII antigen as well as ERG transcription factor.
Contributing Institution:
Veterinary Diagnostic Services, School of Veterinary Medicine
College of Medical, Veterinary and Life Sciences, University of Glasgow
Bearsden Road
G61 1QH
Glasgow, United Kingdom
JPC Diagnosis: Heart arterioles: Atypical endothelial and pericyte proliferation (angioendotheliomatosis), diffuse, severe, with cardiomyocyte degeneration, necrosis, and myocardial fibrosis.
JPC Comment: The contributor excellently summarizes the available literature on this rare condition of cats. Since 1985, the veterinary literature is limited to a total of 15 cases, not including two WSC submissions during this time (WSC 2008 Conf. 3 Case 2 and WSC 2014 Conf. 21, Case 3). Since the submission of this case, a single case report was published on this particular entity.17
Immunohistochemical results for this condition have been published in several articles on this condition in the cat,5,11,17 with intraluminal spindle cells staining strongly positive for Factor-VIII related antigen and VWF, and occasionally for smooth muscle actin (suggesting that some of the cells within the proliferation are of pericyte origin.) Immunohistochemical stains for FVIIIra and smooth muscle actin run at the JPC are in agreement with these findings. Because of the dual cell population, lesions of FSRA are considered to be an aberrant reactive process rather than a neoplasm.
The contributor also points outs potential confusion in the naming of this particular condition. A very different condition, intravascular lymphoma, is presented in the veterinary literature as “malignant angioendotheliomatosis” (aka “intravascular lymphomatosis” and “angiotropic large-cell lymphoma”). While the syndrome of FRSA does bear a similar morphology to the human disease of reactive endotheliomatosis (which in humans is restricted to the skin), there does not appear to be a human precedent for the unfortunate name of “malignant angioendotheliomatosis.)
References:
- Beerlage C, Varanat M, Linder K, Maggi RG, Cooley J, Kempf VA, Breitschwerdt EB. Bartonella vinsonii subsp. berkhoffii and Bartonella henselae as potential causes of proliferative vascular diseases in animals. Med Microbiol lmmunol. 2012; 201(3):319-326.
- Breitschwerdt EB, Maggi RG, Varanat M, Linder KE, Weinberg G. Isolation of Bartonella vinsonii subsp. berkhoffii genotype II from a boy with epithelioid hemangioendothelioma and a dog with hemangiopericytoma. J Clin Microbial. 2009; 47 (6): 1957-1960.
- Breshears MA, Johnson BJ. Systemic reactive angioendotheliomatosis-like syndrome in a steer presumed to be persistently infected with bovine viral diarrhea virus. Vet Pathol. 2008; 45 (5): 645-649.
- Dunn KA, Smith KC, Blunden AS. Fatal multisystemic intravascular lesions in a cat. Vet Rec. 1997; 140(5): 128-129.
- Fuji RN, Patton KM, Steinbach TJ, Schulman FY, Bradley GA, Brown TT, Wilson EA, Summers BA. Feline systemic reactive angioendotheliomatosis: eight cases and literature review. Vet Pathol. 2005; 42(5): 608-617.
- Lapointe JM, Higgins RJ, Kortz GD, Bailey CS, Moore PF. lntravascular malignant T-cell lymphoma (malignant angioendotheliomatosis) in a cat. Vet Pathol. 1997; 34(3): 247-250
- Lazova R, Slater C, Scott G. Reactive angioendotheliomatosis. Case report and review of the literature. Am J Dermatopathol. 1996; 18 (1 ): 63-69.
- Lin BT, Weiss LM, Battifora H. lntravascularly disseminated angiosarcoma: true neoplastic angioendotheliomatosis? Report of two cases. Am J Surg Pathol. 1997 21(10): 1138-1143.
- McDonough SP, Van Winkle TJ, Valentine BA, vanGessel YA, Summers BA. Clinicopathological and immunophenotypical features of canine intravascular lymphoma (malignant angioendotheliomatosis). J Comp Pathol. 2002; 126 (4): 277-288.
- McMenamin ME, Fletcher CD. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002; 26 (6): 685-697.
- Rothwell TL, Xu FN, Wills EJ, Middleton DJ, Bow JL, Smith JS, Davies JS. Unusual multisystemic vascular lesions in a cat. Vet Pathol. 1985; 22(5): 510-512.
- Straumann Kunz U, Ossent P, Lott-Stolz G. Generalized intravascular proliferation in two cats: endotheliosis or intravascular pseudoangiosarcoma? J Comp Pathol. 1993; 109 (1): 99-102.
- Varanat M, Maggi RG, Linder KE, Horton S, Breitschwerdt EB. Cross-contamination in the molecular detection of Bartonella from paraffin-embedded tissues. Vet Pathol. 2009; 46 (5): 940-944.
- Varanat M, Maggi RG, Linder KE, Breitschwerdt EB. Molecular prevalence of Bartonella, Babesia and hemotropic Mycoplasma sp. in dogs with splenic disease. J Vet Intern Med. 2011; 25 (6): 1284-1291
- Wick MR, Rocamora A. Reactive and malignant "angioendotheliomatosis": a discriminant clinicopathological study. J Cutan Pathol. 1988; 15 (5): 260-271.
- Yager JA, Best SJ, Maggi RG, Varanat M, Znajda N, Breitschwerdt EB. Bacillary angiomatosis in an immunosuppressed dog. Vet Dermatol. 2010; 21 (4): 420-428
- Yamamoto S, Shimoyana Y, Haruyama T. A case of feline systemic reactive angioendotheliomatosis. J Fel Med Surg 2015; doi: 10.1177/2044116915579684.