CASE 1: 17-13-2 JT75 (4140283-00)
Signalment:
Adult, male Rhesus macaque (Macaca mulatta)
History:
This Rhesus macaque was part of a study to evaluate different regimens of supportive care after intramuscular exposure with Ebola virus. Intensive supportive care provided to this animal included intravenous fluids, antibiotics and corticosteroids. Despite supportive care, this animal died fourteen days after viral exposure.
Gross Pathology:
At necropsy, there was a focally extensive area of mucosal hemorrhage of the descending colon, approximately 10 cm from the anus. There were multifocal, focally extensive areas of mucosal hemorrhage in the cecum, gastric fundus and proximal duodenum. The liver was diffusely swollen and friable. Clear yellow fluid was present in the pericardial sac, thoracic cavity, abdominal cavity and the scrotum.
Laboratory results:
N/A
Microscopic description:
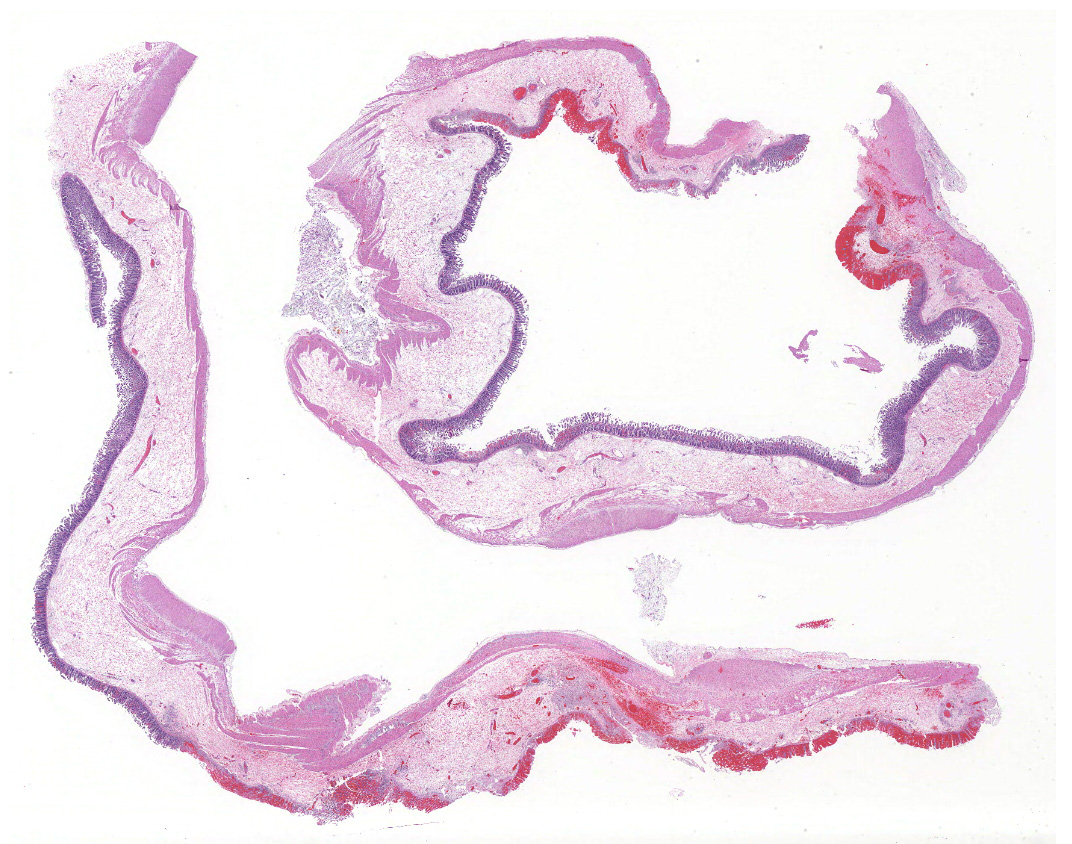
Colon, two sections:
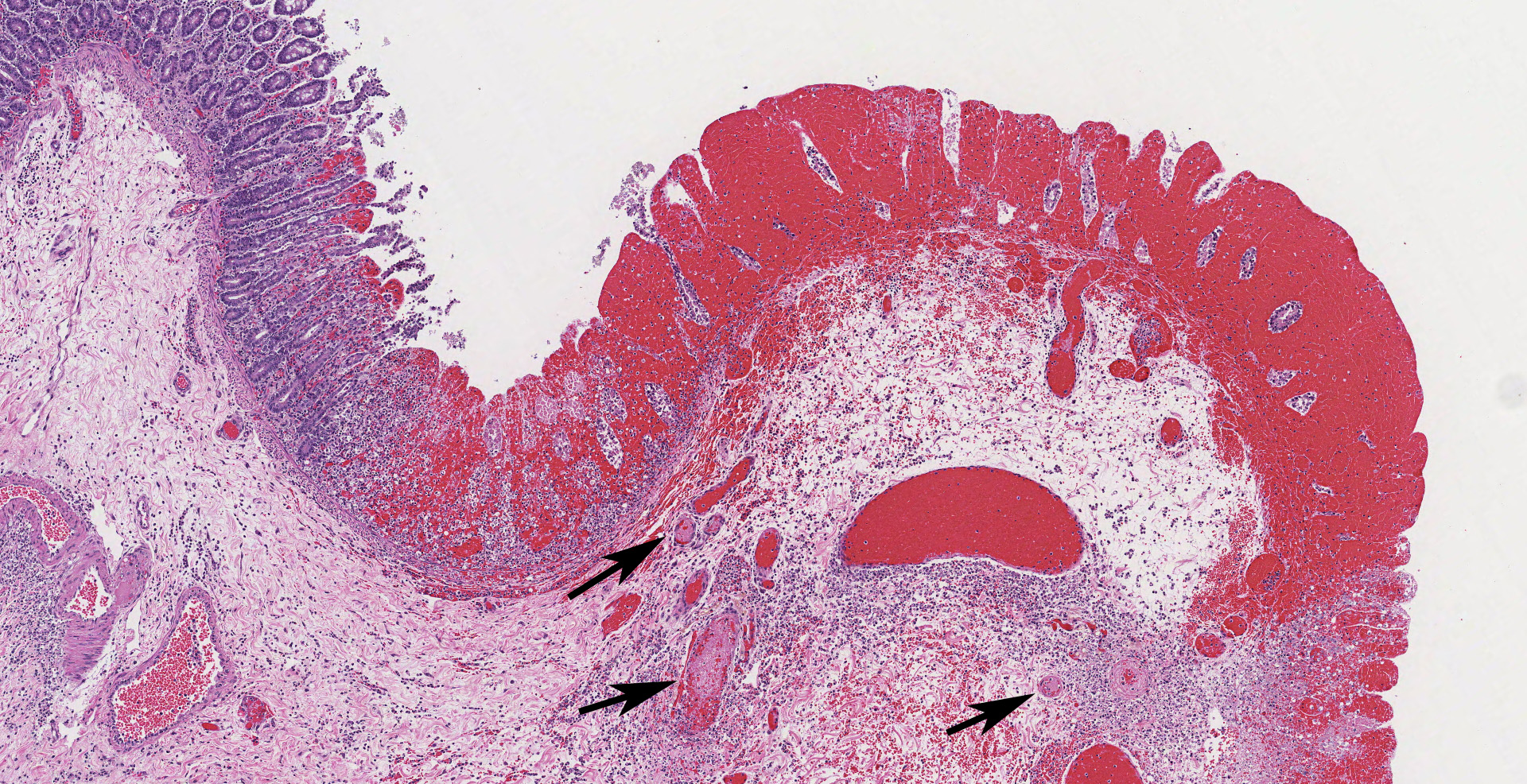
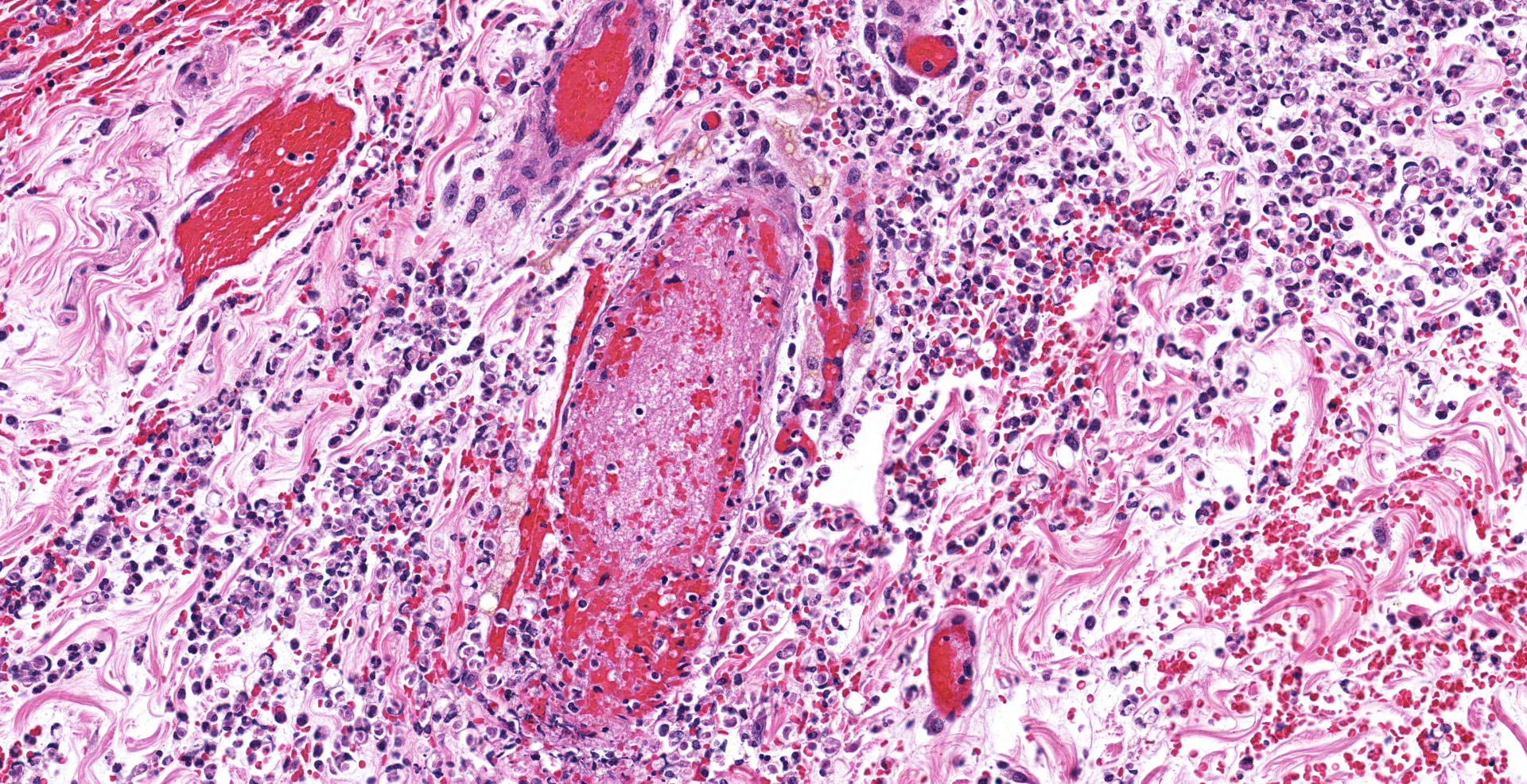
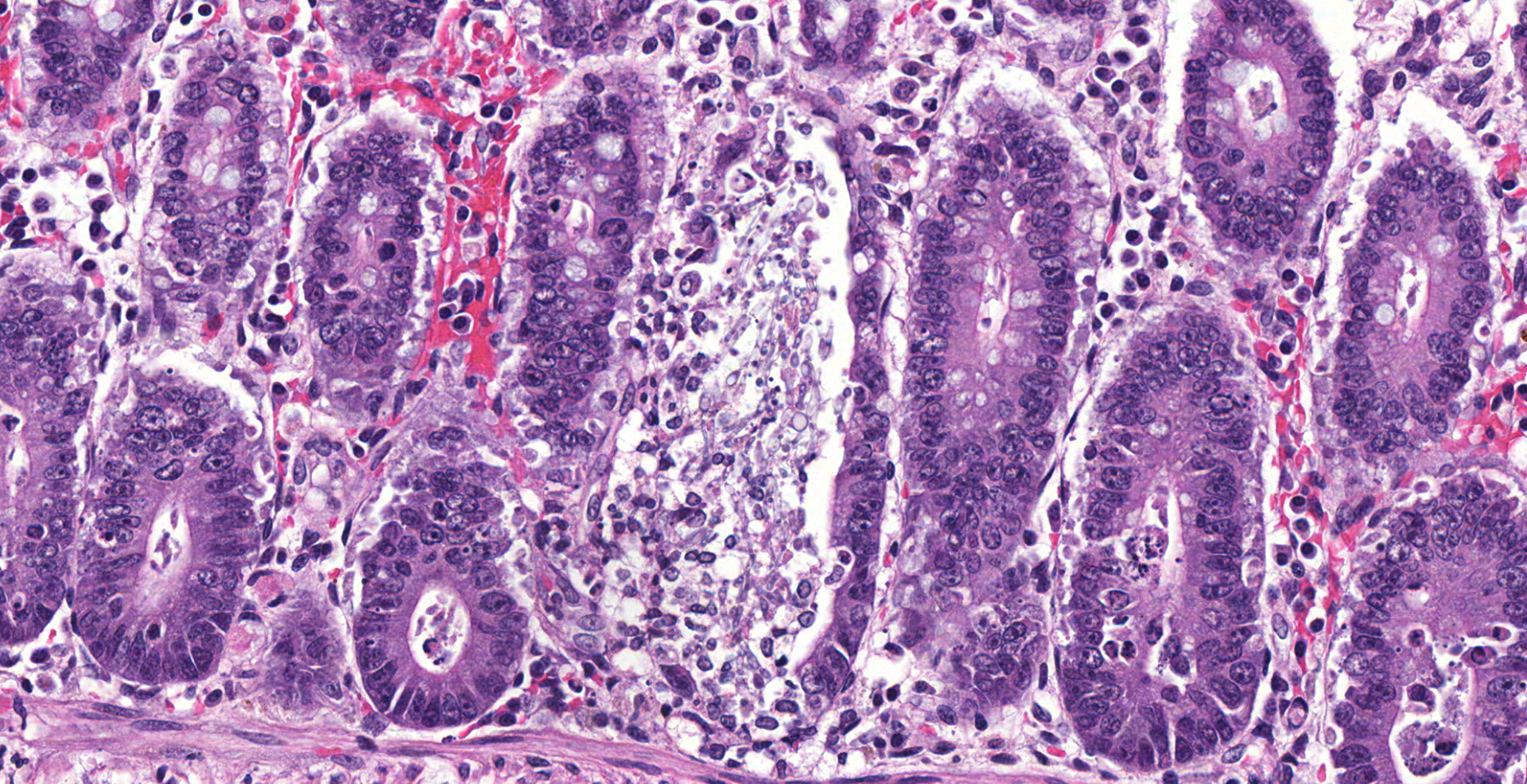
The colonic wall is diffusely and markedly expanded by edema, multifocal hemorrhages and fibrin, and there are several focally extensive areas of mucosal hemorrhage. There are multifocal areas of hemorrhage in which there is a loss of differential staining of the mucosa with retention of tissue architecture (infarcts) and underlying mural blood vessels often contain fibrin thrombi. At higher magnification, there is a transmural inflammatory infiltrate composed primarily of neutrophils, with fewer lymphocytes, plasma cells and macrophages.
Multifocally, within the apical colonic crypt openings and often extending into the lamina propria, there are mats of fungal hyphae admixed with yeast-like blastospores and blastoconidia arranged in short chains (pseudohyphae). Hyphae are 3-6 µm wide, septate and have parallel walls. Blastospores are 2-6 µm in diameter with occasional budding. The yeast forms are stained black on a Grocott's Methenamine silver stain.
Multifocally and frequently within the interstitial layers of the tunica muscularis, there are high numbers of protozoa with a distinct absence of a surrounding inflammatory reaction. These protozoa are ovoid or occasionally piriform and measure 6-12 µm in diameter with a single basophilic nucleus and are stained magenta on a Periodic acid-Schiff stain.
There is a lack of gut-associated lymphoid tissue (lymphoid depletion) and multifocally within the submucosa and lamina propria there is scattered apoptotic cellular debris (lymphocytolysis). In infarcted areas, there are numerous small colonies of mixed bacteria (opportunistic commensal bacterial overgrowth).
By immunohistochemistry, there is intracytoplasmic immunoreactivity to Ebola viral antigen within macrophages and spindle cells and within the lamina propria, there is immunoreactivity of extracellular material (fibrin and serum).
Contributor's morphologic diagnosis:
- Colon: Infarcts, multifocal, with transmural edema, multifocal hemorrhage and intravascular fibrin thrombi.
- Colon: Colitis, marked with mucosal fungal hyphae, blastospores and pseudohyphae (Candida species) and myriad protozoa.
- Colon, gut-associated lymphoid tissue: Necrosis/apoptosis and depletion, diffuse, marked.
Contributor's comment:
Human viral hemorrhagic fevers (VHF) are typically caused by members of the four families: (1) Arenaviridae (several mammarenaviruses), (2) Bunyaviridae (several hantaviruses, nairoviruses, phleboviruses), (3) Filoviridae (certain ebolaviruses, marburgviruses) and (4) Flaviviridae (several flaviviruses sensu stricto).9 VHFs are characterized by severe febrile illness with bleeding diathesis that culminated in shock and multiorgan failure. Key features in the pathogenesis of VHFs include coagulopathy, tissue necrosis and immune suppression. These processes occur via activation of the coagulation cascade, massive release of cytokines and direct cellular lysis, most notably of lymphocytes.
Filoviruses (family Filoviridae) include the Ebola virus (including Zaire, Sudan and Kikwit species/strains, among others) and Marburg virus genera and Reston virus which causes disease in nonhuman primates, but not in humans. The most consistent gross lesions in nonhuman primates experimentally infected with Ebola virus by either intramuscular or aerosol exposure include a red macular skin rash; a friable, pale liver; enlarged, firm spleen and lymph nodes; and hemorrhage and congestion of the pylorus, proximal duodenum and other segments of the gastrointestinal tract. Histologic lesions typically include hepatic necrosis and inflammation; lymphoid necrosis in the spleen, lymph nodes and other lymphoid tissue; fibrin that fills the splenic sinusoids; and intravascular fibrin thrombosis in the renal papilla, gastrointestinal tract and many other sites.10,12 Other histologic findings in this animal included all of those listed above, as well as myocardial infarctions with myocardial necrosis.
Nonhuman primates infected with Ebola virus typically succumb to disease within 6-10 days following exposure. This animal lived slightly longer than average, perhaps due to supportive care; however, immune suppression in this animal was undoubtedly exacerbated by the administration of corticosteroids that allowed unchecked proliferation of commensal fungi and protozoa in the gastrointestinal tract. Corticosteroids, either endogenous or exogenous, can cause decreased immune responsiveness, suppression of inflammation and delayed wound healing.2
The histomorphologic and electron microscopic (EM) features of the colonic fungus are consistent with a species of Candida. Candida sp. are dimorphic fungi found in the environment, typically in the yeast form. After ingestion or inhalation, these yeasts can persistently reside on the mucosa of the gastrointestinal tract as a nonpathogenic commensal in immunocompetent animals. In the event of an immunocompromising event, these yeasts can shift to hyphal and pseudohyphal forms and proliferate on mucosal surfaces.11 Typical manifestations of candidiasis in humans and animals, particularly young animals, occur on keratinized epithelial surfaces such as the skin, oral cavity, esophagus, rumen or vaginal mucosa. Systemic candidiasis can occur in any tissue, including the gastrointestinal tract, central nervous system and urinary systems.
Scanning and transmission EM was used to better characterize the protozoa. EM images identified the presence of 4-5 flagella protruding from the posterior pole, one of which is attached to the body wall forming an undulating membrane (image). The size, shape, presence of flagella and the single nucleus are consistent with a species of Pentatrichomonas.4 Trichomonads are generally nonpathogenic commensal organisms found in many species of mammals and birds. As with other commensals, opportunistic infections can occur in cases of immune suppression or other disease conditions, and gastrointestinal trichomoniasis, specifically gastritis, has been reported in Rhesus macaques infected with simian immunodeficiency virus.1,6
Contributing Institution:
United States Army Research Institute of Infectious Disease
Pathology Division
1425 Porter Street
Fort Detrick, MD 21702-5011
JPC diagnosis:
1. Colon, submucosal vessels: Vasculitis, necrotizing, multifocal, moderate with thrombosis, mucosal infarction, and severe submucosal edema.
2. Colon: Colitis, neutrophilic and histiocytic, multifocal and transmural, marked with intra- and extracellular trichomonads.
3. Colon: Colitis, neutrophilic and histiocytic, multifocal, moderate, with infiltrating yeasts and pseudohyphae.
JPC comment:
Ebola virus infection is a current and relevant topic of discussion, with periodic outbreaks most recently affecting African countries such as the Democratic Republic of the Congo, the Republic of South Sudan, the Republic of Uganda, Guinea, Liberia, and Sierra Leone.3 As of 13 September 2020, the current epidemic (11th) of Ebola virus (Zaire strain) disease in the Equateur Province of the Democratic Republic of the Congo has claimed the lives of 48 people, with 53 recovering. The approximately 50% mortality rate is common and may reach nearly 90% case fatality rates, and this particular province experienced a previous outbreak just two years prior. Resources for testing, medical providers, and medical supplies remains a challenge in these countries, with the World Health Organization contributing financially, and by providing healthcare workers.7
During the Ebola outbreaks among humans and great apes in Gabon and the Democratic Republic of the Congo from 2001 to 2003, numerous small vertebrates were captured and sampled in an effort to identify a natural host or reservoir host. The only animals implicated were a small number of fruit bats who either were serologic positives, or PCR positives with Ebola virus fragments present. However, no bats were serologic and PCR positive, and to date, there has been no successful virus isolation from a bat. Most evidence supports the hypothesis that bats are the likely natural host for Ebola virus, but they may also be incidentally infected by a different animal, the true natural host.5 The book Spillover (2012) discusses considerations of emerging disease investigation, humanity's clash with nature, and the spillover of zoonotic diseases from wild animal populations into human populations.8
Ebola virus initially replicates in antigen-presenting cells, such as macrophages and dendritic cells, but may also infect numerous other cell types such as fibroblastic reticular cells, blood monocytes, hepatocytes, Kupffer cells, and cells of the adrenal gland and testicle. Peripheral mononuclear cells with ebolaviral infection fail to produce type 1 IFNs and inhibits IFN-a production through disruption of the RIG-1 pathway and Dicer-dependent protein kinase R. STAT proteins are also inhibited, blocking transcription of antiviral genes. In the later stages of disease, numerous cytokines (IL1b, IL-1RA, IL-6, IL-8, IL-10, IL-15, IL-16, TNF-a), chemokines, and growth factors (MIP-1a, MIP-1b, MCP-1, M-CSF, MIF, IP-10, GRO-a, and eotaxin) are increased from 5-1000 times normal, creating a cytokine storm resulting in organ failure, sepsis syndrome, and death.3
References:
1. Blanchard JL and Baskin GB. Trichomonas Gastritis in Rhesus Monkeys Infected with the Simian Immunodeficiency Virus. J Infect Dis 1988; 157:5, pp. 1092-1093.
2. Ferguson DC and Hoenig M. Chapter 11: Endocrine System. In: Duncan and Prasse's Veterinary Laboratory Medicine: Clinical Pathology, 5th Ed. Jon Wiley and Sons Ltd., Chichester, UK, 2011, pp. 318.
3. Furuyama W, Marzi A. Ebola Virus: Pathogenesis and Countermeasure Development. Annu. Rev. Virol. 2019;6:435-458.
4. Gardiner CH, Fayer R and Dubey JP. In: An Atlas of Protozoal Parasites in Animal Tissues, 2nd Ed. Armed Forces Institute of Pathology, Washington, DC, 1998: pp.6-7.
5. Han HJ, Wen HL, Zhou CM, et al. Bats as reservoirs of severe emerging infectious diseases. Virus Research. 2015;205:1-6.
6. Kondova I, Simon NA, Klumpp A, et al. Trichomonad Gastritis in Rhesus Macaques (Macaca mulatta) Infected with Simian Immunodeficiency Virus. Vet Pathol 2005; 42:1, pp. 19-29.
7. ProMED-mail. Ebola update (47): Congo DR (EQ). ProMED-mail 2020; 22 Sep: 20200922.7803256. http://www.promedmail.org. Accessed 24 September 2020.
8. Quammen D. Spillover. New York, NY: W.W. Norton & Company, Inc. 2012.
9. Radoshitzky SR, Bavari SA, Jahrling PB and Kuhn JH. Chapter 23: Filoviruses. In: Medical Aspects of Biological Warfare. Office of the Surgeon General; Falls Church, VA 2018; pp. 569-571.
10. Twenhafel NA, Mattix ME, Johnson JC, et al. Pathology of Experimental Aerosol Zaire Ebolavirus Infection in Rhesus Macaques. Vet Pathol 2013; 50: 514
11. Zachary JF. Chapter 4: Mechanisms of Microbial Infections. In: Pathologic Basis of Veterinary Disease, 6th Ed. Springer, St. Louis, MO, 2017, pp. 232-233.
12. Zumbrun EE, Bloomfield HA, Dye JM, et al. A Characterization of Aerosolized Sudan Virus Infection in African Green Monkeys, Cynomolgus Macaques and Rhesus Macaques. Viruses 2012, 4, 2115-2136.