CASE 1: N16-032 (4084301-00)
Signalment: 13 yrs of age, spayed female, Golden Retriever, Canis lupus familiaris, canine.
History:
It was reported that the patient had decreased appetite and lethargy for the past 1-2 weeks and had diarrhea for ~1 day. She had a history of seizures for the past ~3 years; her last seizure was 1 month ago. The patient had a generalized lymphadenopathy (lymphoma). Patient was on phenobarbital, was obese and was 5-7% dehydrated.
Gross Pathology:
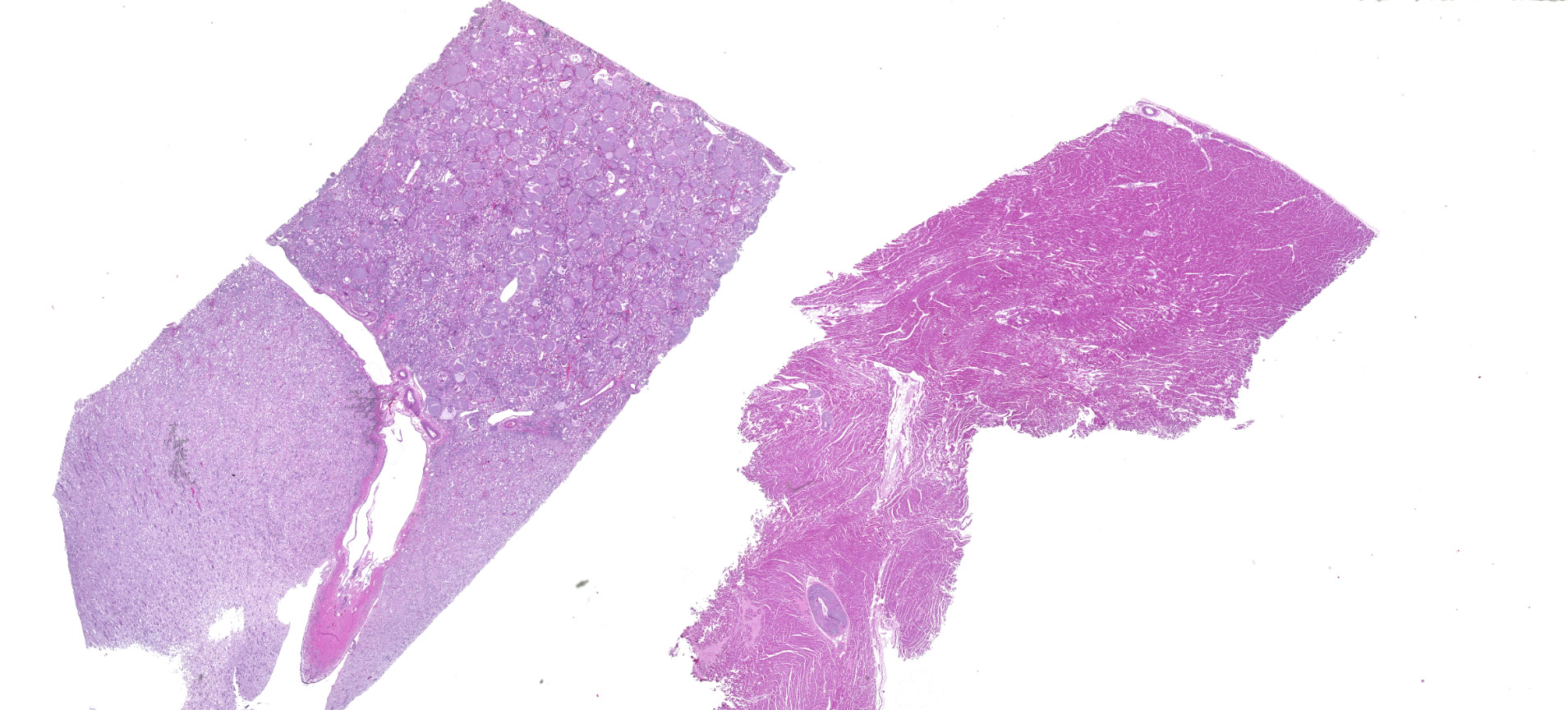
Relevant gross pathologic findings included: Multiple (n=10) well-demarcated, unattached, soft, white, lipid-like masses (lipomas) ranging from 4-20 cm in diameter, were present in the subcutis. There was generalized enlargement of peripheral lymph nodes ~4 times larger than normal. The left caudal lung lobe had a 1 cm focal firm, dark red area of depression. The right ventricular free wall was 3 mm and the left was 7 mm (right:left ratio of 1:2.3). The right and left kidney contained innumerable miliary white foci throughout the cortical parenchyma. A 3mm cyst was present on the cortical surface of the right kidney. The parietal lobe of the left cerebral hemisphere was depressed by an approximately 2.2x1.5x2 cm firm mass originating from the dura mater (meningioma).
Laboratory results:
Cytology: It was reported that ante-mortem cytology of blood smears from this animal were consistent with lymphoid leukemia.
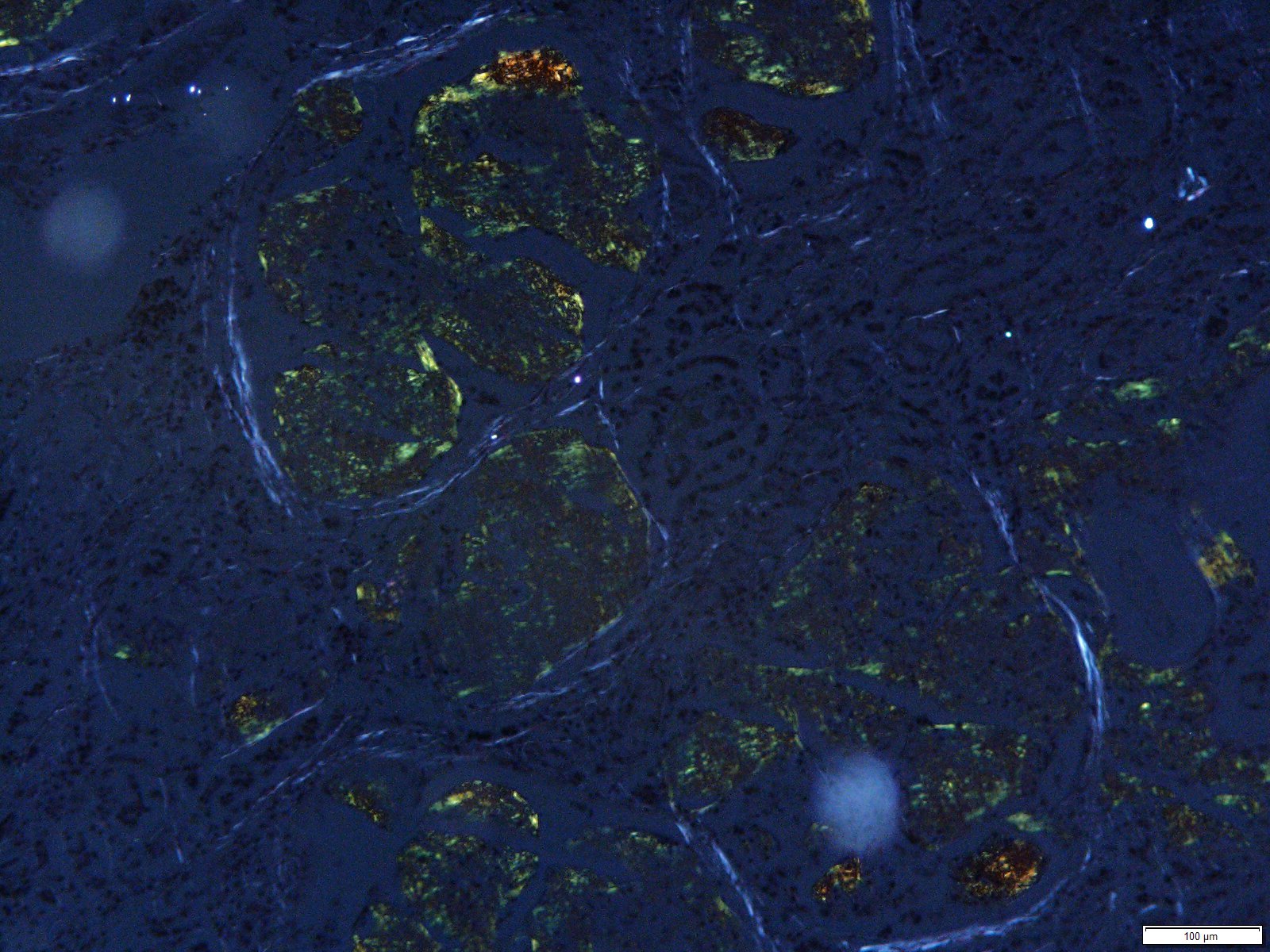
Special staining: Under polarized light, Congo Red special stain revealed apple-green birefringence of material effacing glomeruli and cardiac vessel.
Microscopic description:
The microscopic findings for the submitted tissues are:
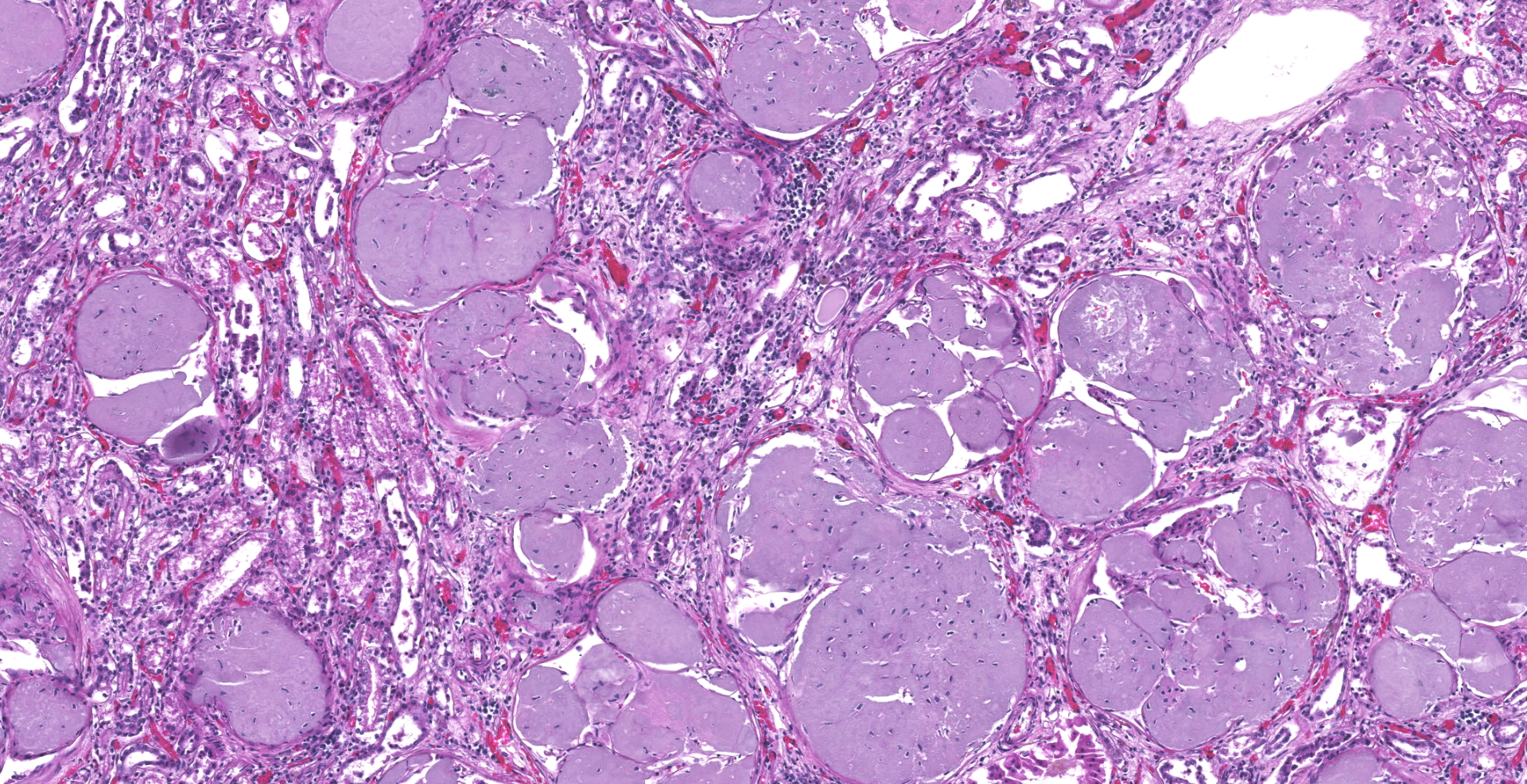
Kidneys: Glomeruli are diffusely and globally obscured and expanded by amorphous homogenous pale eosinophilic to amphophilic material (amyloid). Bowman's capsules are variably thickened and there is marked synechia formation. The interstitium is diffusely infiltrated with small to moderate amounts of lymphocytes and plasma cells.
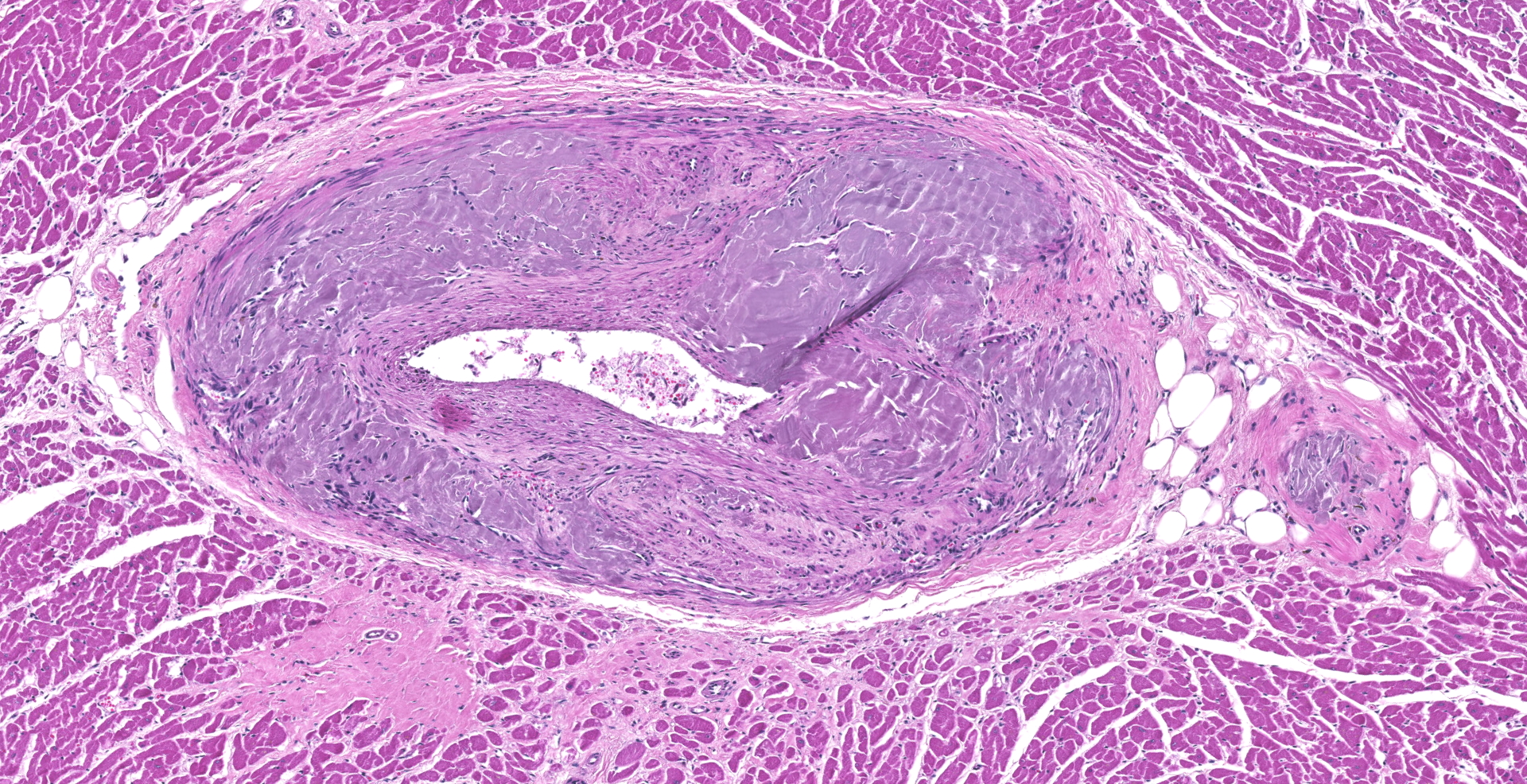
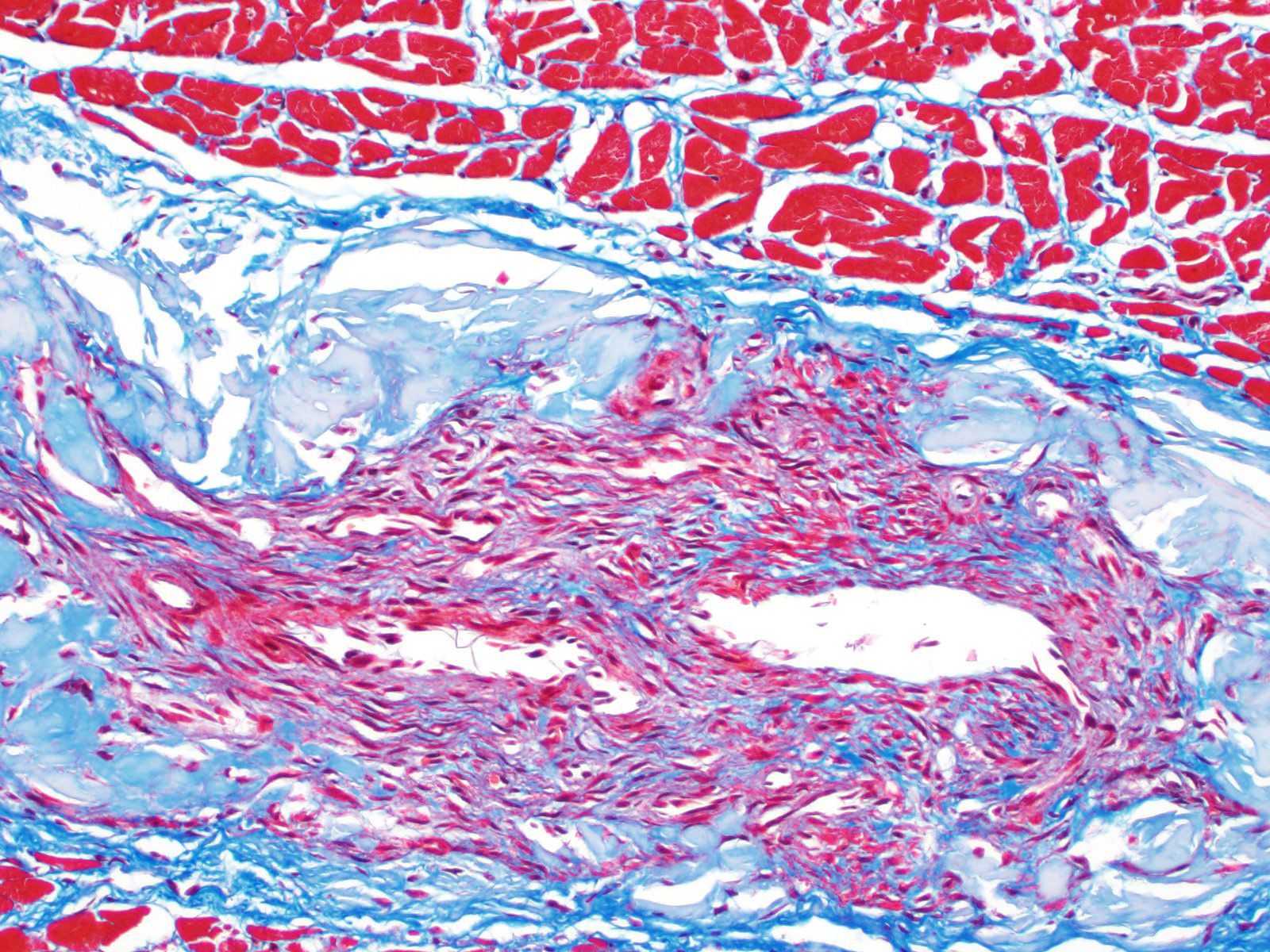
Heart: Arteries and arterioles throughout the myocardium frequently have transmural thickening of the vessel wall by amorphous pale amphophilic material (amyloid). Affected vessels often have recanalization characterized by multiple small caliber spaces. There is marked endocardial fibrosis and few scattered multifocal areas of mild to moderate interstitial fibrosis; associated cardiac myocytes are shrunken and fragmented (degeneration). There are also a few multifocally extensive areas of myocardial loss and replacement by adipose tissue and scattered macrophages, lymphocytes and plasma cells.
Contributor's morphologic diagnosis:
Kidney: Severe diffuse global glomerular amyloidosis
Heart: Submassive severe coronary arterial wall amyloidosis with recanalization
Heart: Moderate multifocal interstitial myocardial fibrosis and fatty infiltration with myocardial degeneration.
Morphologic diagnoses for tissues not submitted:
Meninges: Meningioma
Brain: Meningioma-associated cerebrocortical compression with locally extensive astrocytosis, oligodendrogliosis, neuronal satellitosis and periglial edema
Lymph node and Spleen: Metastasis of lymphoid leukemia
Lung: Focal bronchioloalveolar carcinoma, well-differentiated
Contributor's comment:
The animal in this case had multiple neoplastic processes. The neoplastic leukemic lymphoid cells were effacing the lymph nodes, in pericapsular vessels associated with lymph nodes and multifocally present in the spleen. This neoplasm is considered the primary cause of illness in this case. The meningioma and its compression against the parietal lobe of the brain resulted in seizures. There was also a well-differentiated bronchioloalveolar carcinoma identified in one site in the lung.
Amyloidosis can be secondary to chronic inflammatory or neoplastic processes; considering the multiple neoplasms in this case, including lymphoid leukemia, it is likely that the amyloid deposition in the kidney, and possible the myocardial vasculature as well, was secondary to other disease processes going on. The amyloid deposition in the vessels of the heart may also be secondary to aging, as senile amyloid plaques have been reported in muscular arteries of the myocardium, lungs, and spleen of old dogs. The cause of the myocardial lesions may be secondary to the vascular amyloidosis in the heart and impaired oxygenation of the associated musculature.
The term amyloid refers to a group of glycoproteins whose protein components represent ?-pleated sheet patterns. In a healthy patient, the immune system is usually able to either repair (chaperones) or degrade (ubiquitin-proteosome pathway) these proteins. However, if the body is unable to perform such feats, one can end up with amyloidosis. These proteins deposit into organs and result in pressure atrophy of adjacent cells as they accumulate.
Amyloidosis can be classified into systemic (amyloid deposits in multiple organs) and localized (single organ) amyloidosis, and further categorized into primary and secondary amyloidosis. Primary amyloidosis, which is associated with the amyloid light chain (AL form), is the most common form in humans, while secondary amyloidosis, which is associated with the amyloid-associated (AA) form, is most common in animals.
The AL form is immunoglobulin-derived and can be deposited by B lymphocytes as well as plasma cells and result from immunocyte dyscrasias. The AA form of amyloidosis is derived from the precursor serum-amyloid associated (SAA) protein. This precursor is synthesized in the liver and its production is increased in pro-inflammatory states such as chronic inflammation and neoplasia. With the lack of proteolytic action on these proteins, amyloidosis can result. Humans have these same two forms, as well as the ?-amyloid form (A?) which is found in the cerebral plaques of Alzheimer patients. Other less common forms of amyloidosis in animals is islet amyloid polypeptide (IAPP) produced in pancreatic islets of cats with non-insulin-dependent diabetes mellitus and apolipoprotein AI (apoAI) amyloidosis deposited in pulmonary vessels of aged dogs.
The kidney is the most common site for deposition of AA amyloid in association with other disease. In dogs, localization to glomeruli is most common. In contrast, in cats and Chinese Shar-Pei dogs with renal amyloidosis, the amyloid is usually localized to the renal medulla. Clinical pathologic parameters frequently associated with glomerular amyloidosis include proteinuria and may progress to nephrotic syndrome. With medullary amyloidosis, the amyloid becomes obstructive in nature resulting in capillary and tubular basement membrane obstruction and thickening, papillary necrosis and medullary interstitial fibrosis. A hereditary predisposition to AA amyloidosis (familial amyloidosis) has been described in Abyssinian cats and Chinese Shar-Pei dogs.
The amyloid that was found in the cardiac vessels of this animal may be due to the systemic disease affecting this dog but may also be associated to aging. Cardiac amyloidosis has been reported in dogs ?7 years and is somewhat comparable to the senile cardiac amyloidosis of humans, which is seen in those age 70 and greater. A prominent difference is that amyloid builds up in the atria and ventricles of the heart in humans, while studies show that it primarily only affects arterioles in dogs.2
Grossly, amyloidosis can be diagnosed by using Lugol's agent followed by diluted sulfuric acid. If the tissue becomes a purple to dark blue tinge, it is positive for amyloidosis. Microscopically, one could use Congo Red stain as well as thioflavine-T. The stain itself is not taken up by the protein, but rather caught in the ?-pleated sheet. Under polarized light, the amyloid proteins emit an apple-green birefringence with the Congo Red. In the case of AA amyloidosis, the addition of potassium permanganate results in the loss of the Congo Red stain, enabling differentiation from the AL form of amyloidosis. Thioflavine-T is bright yellow under fluorescent light and may be more sensitive than Congo Red. Utilizing electron microscopy, amyloid fibrils are characterized by 7.5-10 nm diameter, nonbranching tubules.
Contributing Institution:
Tuskegee University College of Veterinary Medicine
1200 West Montgomery Road
Tuskegee, AL 36088
http://www.tuskegee.edu/academics/colleges/cvmnah/school_of_veterinary_medicine.aspx
JPC diagnosis:
1. Kidney: Amyloidosis, glomerular, global, diffuse, severe, with tubular degeneration and loss, and chronic interstitial nephritis, golden retriever, canine.
2. Heart:
Amyloidosis, arterial, diffuse, severe, with multifocal myofiber atrophy, loss,
and fibrosis.
JPC Comment: The contributor describes amyloidosis in detail, and the methods of detection remain the most accessible in the field.
Amyloidosis documented in a wide range of wild and captive mammals and birds.5 While there are many fewer documented cases, reptiles with cases of systemic amyloidosis have been documented within the last three years. A 12-year-old male African tiger snake with concurrent Mycobacterium avium-intracellulare complex infection, biliary cystadenocarcinoma, with intrahepatic metastases had splenic amyloidosis with amyloid also present in testicular interstitium and occasional blood vessel walls. Mycobacteriosis is a common cause of reactive amyloidosis, and the serum amyloid A (SAA) gene is highly conserved across species.1
A strain of mouse designated AppNL-G-F/NL-G-F has been bred with three distinct introduced mutations that increase production of amyloid precursor protein (APP), which results in reliable overproduction of amyloid b and histologic and clinical signs consistent with Alzheimer's disease. Based on examination of the non-cognitive, emotional domains of these mice, they serve as a useful model of preclinical Alzheimer's disease in humans.4
Human cardiac light chain (AL) amyloidosis is a particularly lethal disease, responsible for approximately 75% of mortality associated with AL amyloidosis. Zebrafish were altered to selectively secrete human l-light chain proteins in the liver, which were then exported to systemic circulation. These zebrafish developed cardiomyopathies consistent with early cardiac AL amyloidosis, and may represent a future animal model of the human disease.3
References:
1. Burns RE, Gaffney PM, Nilsson KPR, Armien AG, Pessier AP. Systemic amyloidosis in an African Tiger Snake (Telescopus semiannulatus). J Comp Path. 2017;157:136-140.
2. Jonsson, L., Senile cardiac amyloidosis in the dog. Acta Vet Scand, 1974. 15(2): p. 206-18.
3. Mishra S, Joshi S, Ward JE, et al. Zebrafish model of amyloid light chain cardiotoxicity: regeneration vs degeneration. Am J Physiol Heart Circ Physiol. 2019;316(5):H1158-H1166.
4. Sakakibara Y, Sekiya M, Saito T, Saido T, Iijima KM. Cognitive and emotional alterations in App knock-in mouse models of Ab amyloidosis. BMC Neurosci. 2018;19:46.
5. Terio KA, McAloose D, St. Leger J. Pathology of Wildlife and Zoo Animals. Elsevier: San Diego, CA. 2018.