CASE 2: 72739 (4155463-00)
Signalment:
16-week-old female FVB/N mouse (Mus musculus)
History:
50 female FVB/n mice were sedated with ketamine/xylazine and inoculated with Her2-positive mammary gland tumors (MGT) on the 4th right mammary fat pad. All animals were expected to develop MGT, however only 50% of animals did. Those that developed tumors took twice as long as normal to reach experimental manipulation size, and most had tumor regression approximately 2 weeks afterwards. 3/8 control animals had masses in abdominal cavity.
Gross Pathology:
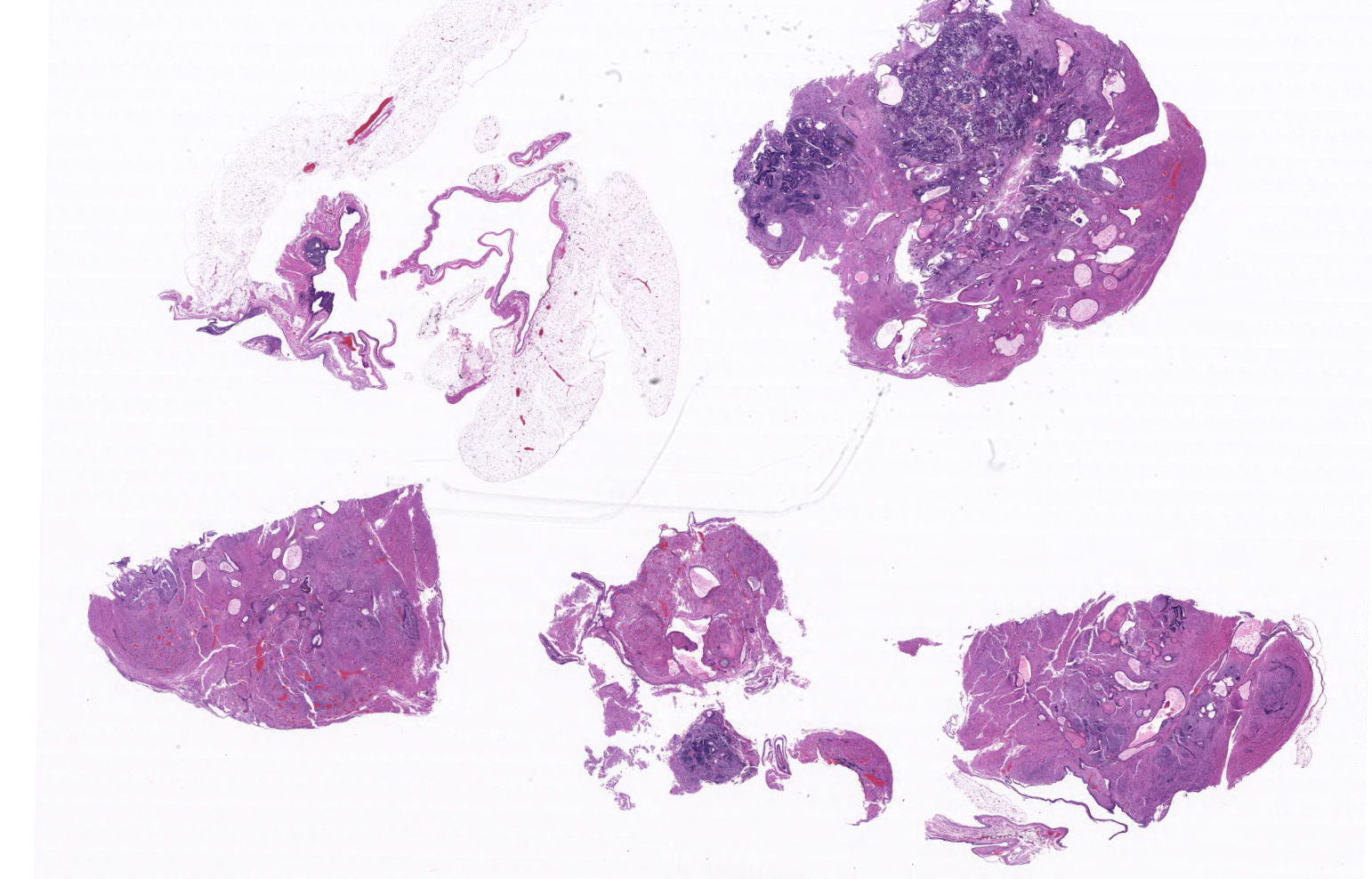
A 1.9 x 1.1 x 1.0 cm round, soft, tan to pink multinodular mass is present in the area of the left ovary.
Laboratory results:
N/A
Microscopic description:
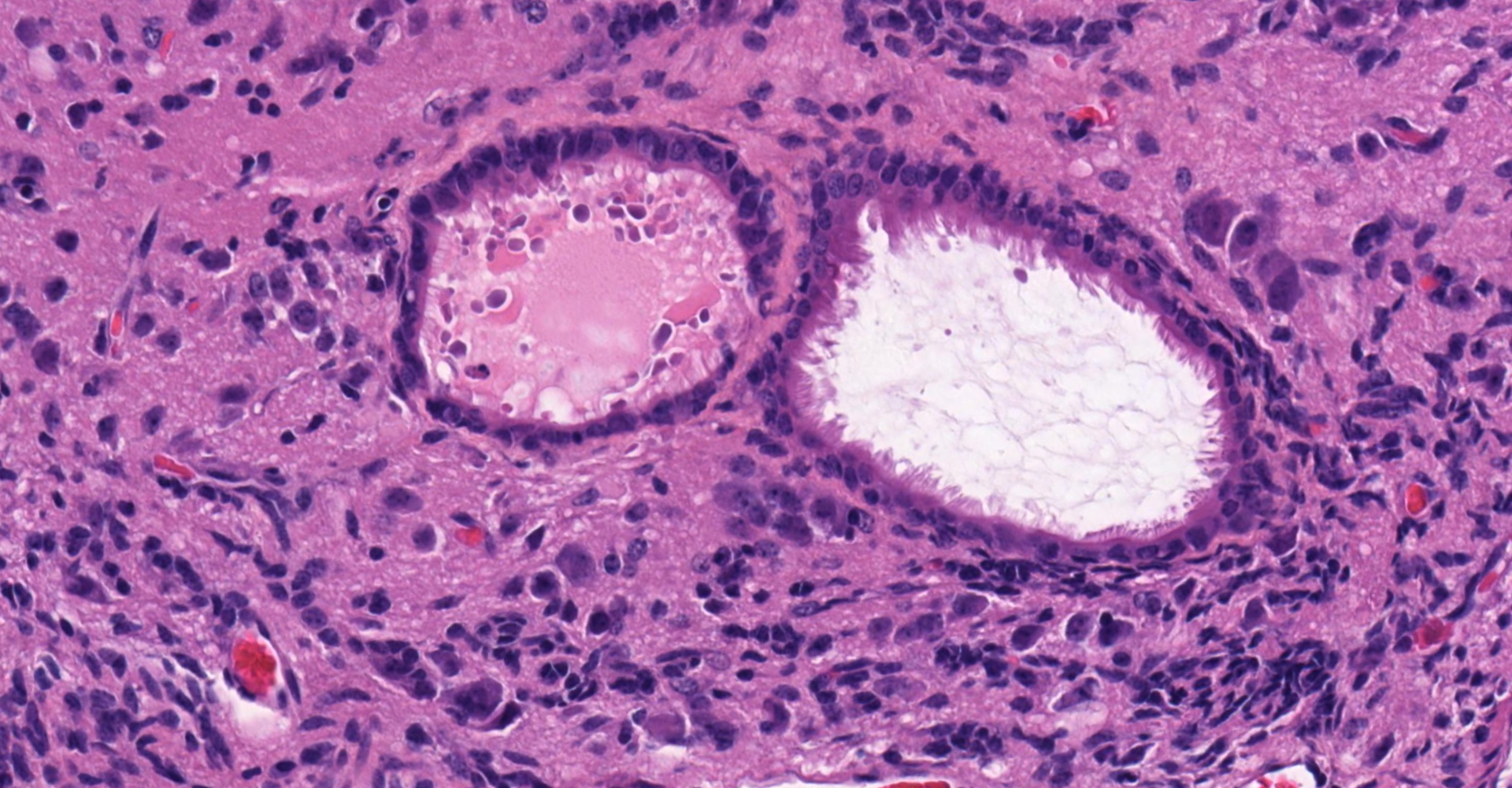
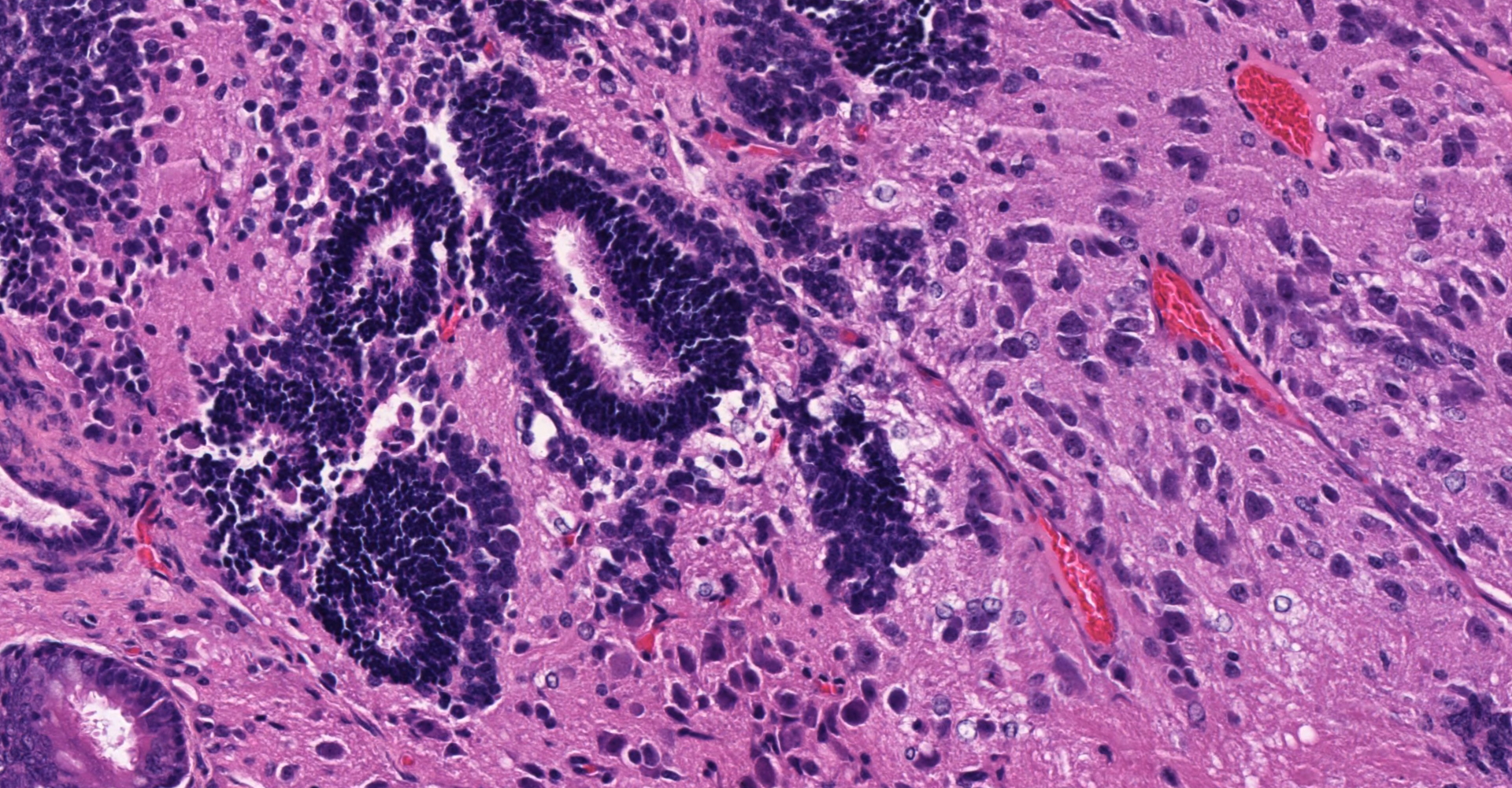
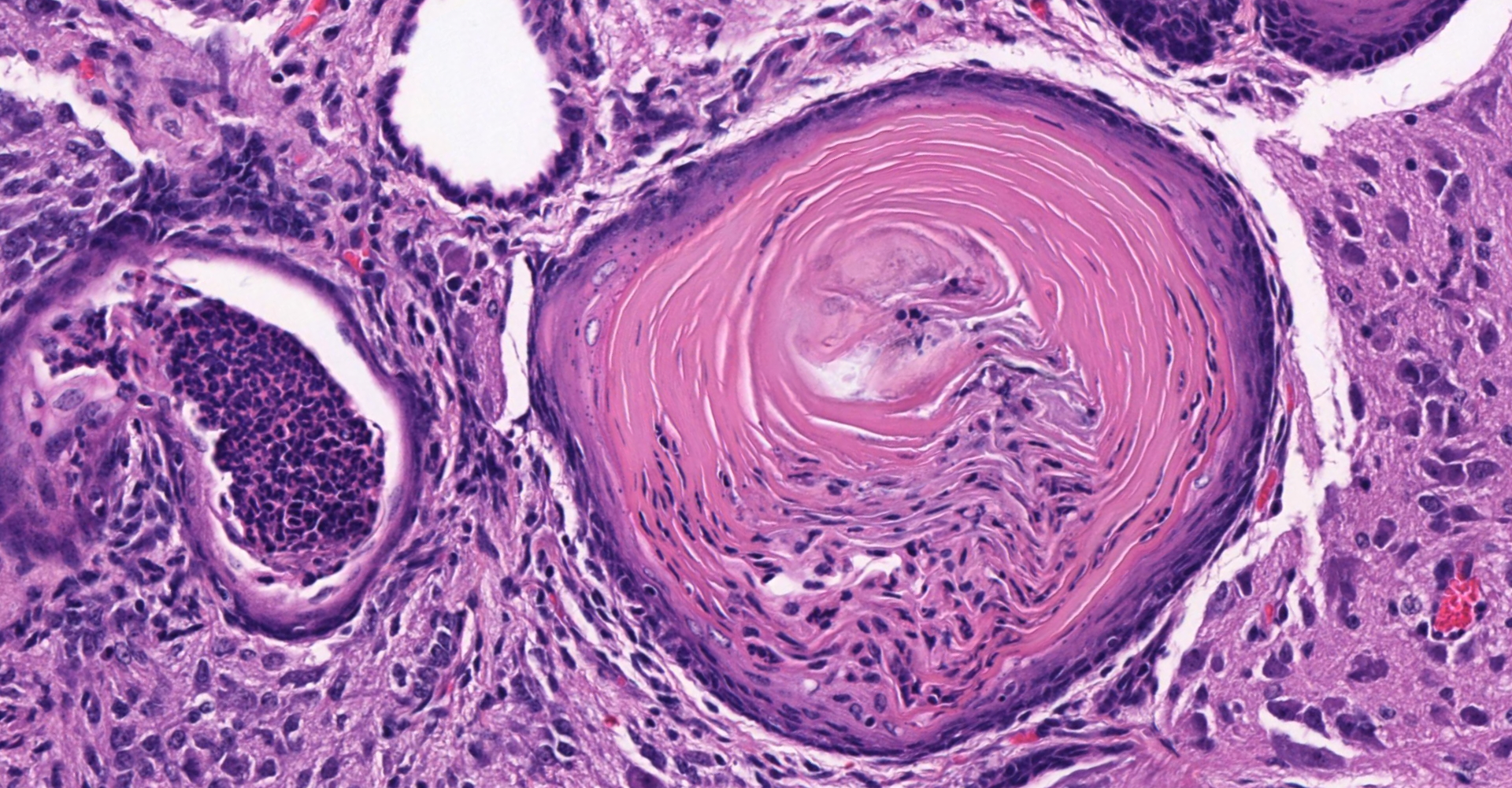
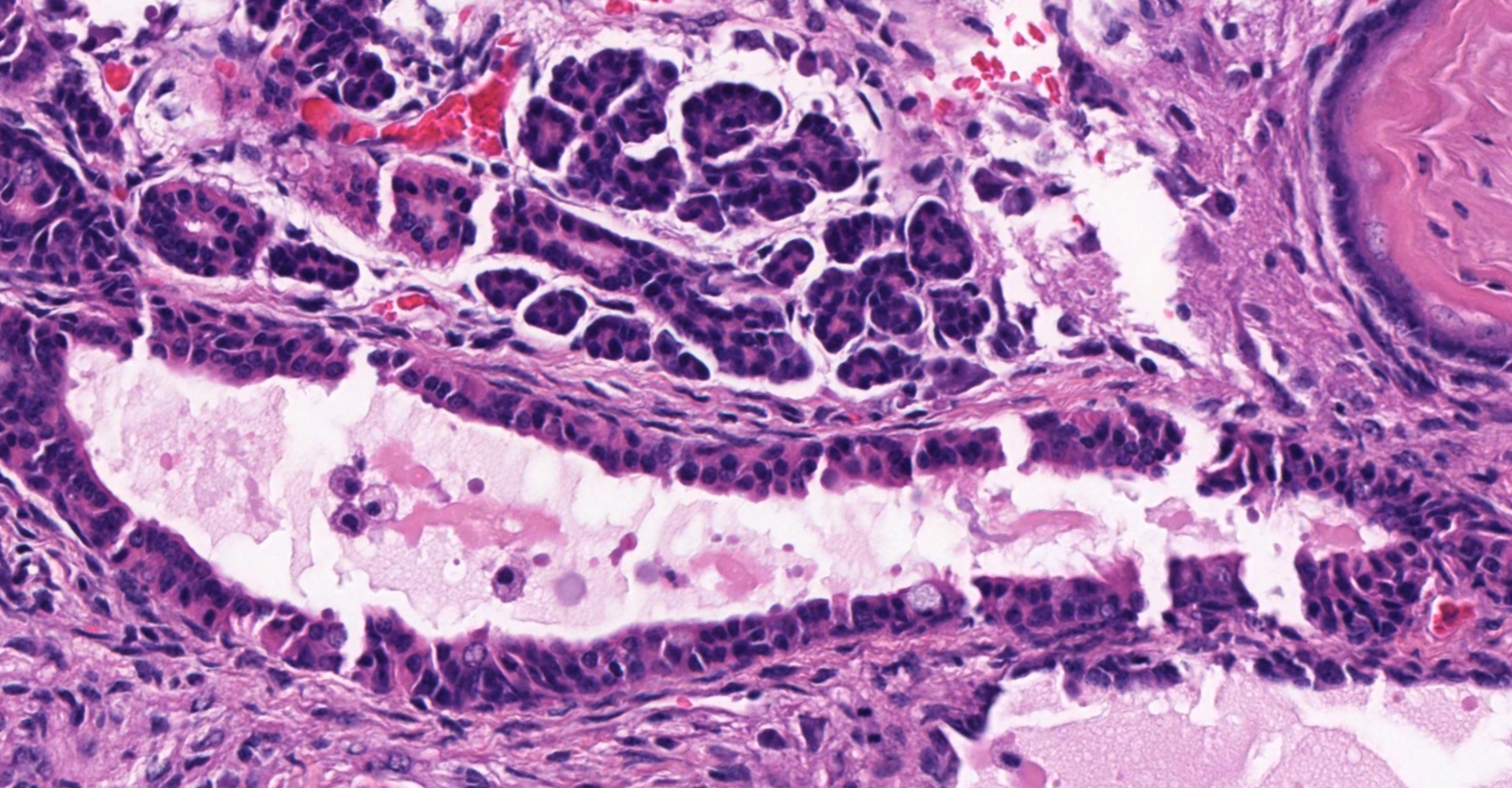
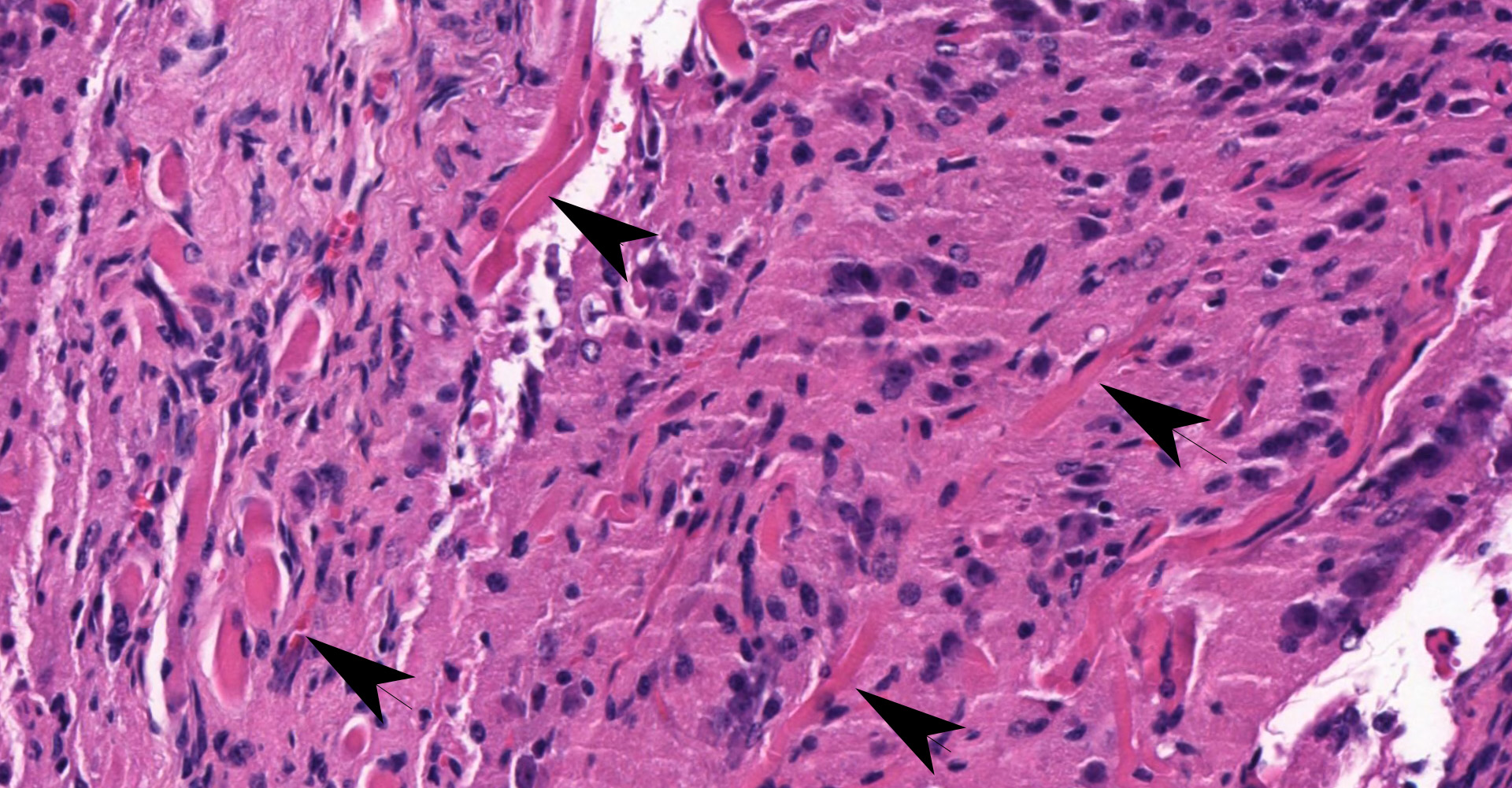
Ovary and oviduct. Effacing greater than 95% of the ovarian tissue is an infiltrative, unencapsulated, poorly demarcated, variably cellular and cystic, neoplasm composed of well-differentiated tissues from three primordial germ cell layers. Ectodermal elements include neural tissue comprised of neurons, glial cells, and clusters of small, hyperchromatic primitive neuroblastic cells embedded in neuropil, and numerous ependymal-lined cysts of varying size, up to 1 mm in diameter. These cysts are lined by columnar, ciliated cells and contain moderate amounts of basophilic material and degenerate cellular debris. Additional ectodermal elements include multiple cysts lined by stratified squamous epithelium with occasional keratohyaline granules that exhibit gradual and abrupt keratinization and contain variable amounts of lamellated keratin, debris, and degenerate cells. Endodermal elements include numerous variably sized cysts up to 3 mm in diameter which are lined by ciliated, pseudostratified columnar epithelium with interspersed goblet cells (respiratory epithelium), and mucous and serous mixed glands of variable maturity, arranged in haphazard acini along a larger duct. Cysts contain variable amounts of basophilic mucinous material to eosinophilic proteinaceous material, with scattered degenerate cells. Mesodermal elements include rare scattered bundles of smooth muscle and scattered foci of variably mature cartilage. Mitotic figures are rare. A few scattered ovarian follicles ranging from primordial to late primary maturity are present on the slide, as well as multiple cross sections of oviduct.
Contributor's morphologic diagnosis:
Ovary: teratoma
Contributor's comment:
Teratomas are a type of germ cell tumor that exhibit somatic cell differentiation with elements of at least two embryonic germ layers (ectoderm, mesoderm, endoderm).1,3 Primordial germ cells must migrate within the embryo to reach the gonads, and as a result teratomas can arise anywhere along this migration path, in addition to presentation within gonadal tissue.14 Teratomas of the ovary are rare in domestic animals but have been reported in many species, most commonly in the bitch and cow.1,11
FVB/N mice express the Fv-1b allele, which allows for susceptibility to the B strain of Friend Leukemia virus. This strain of mice is used for transgenic studies due to traits of super-fecundity, a large pronucleus in fertilized zygotes, and survivability of embryos following injection.10,15 In studies of spontaneous pathology in FVB/N mice, ovarian teratomas have been reported at a rate of 2%, whereas male FVB/N mice are thought to be resistant to testicular teratomas.5,7,10 However, these studies were conducted in aging mice and retired breeders, all at least 14 months of age. In this case, the animals were only 16 weeks old. Tumor frequency in this cohort was at least 4%, potentially higher but unable to be determined due to lack of availability of tissues.
Spontaneous ovarian teratoma development in mice is rare. In order to study the human condition, transgenic mouse models have been generated which have led to genetic associations with teratoma development. These include deficiency in c-mos, a serine kinase that is required for arrest of meiosis II. Lack of this enzyme is associated with development of parthenotes, which are thought to further progress to teratomas.2,4 Bcl-2 is an outer mitochondrial membrane protein that acts to suppress apoptosis. In studies where transgenic mice overexpressed Bcl-2 in ovarian granulosa cells, there was increased teratoma development.4,6 In a mouse model overexpressing alpha and beta chains of human chorionic gonadotropin, teratomas were thought to be a result of progression of luteomas, which occurred with increased frequency.4,8 In a study investigating the role of signal transducer and activator of transcription 1 (STAT1), ovarian teratomas formed in a group of animals with an FVB/N background that were also overexpressing MMTV-neu, the rat homologue of mouse erbB2 oncogene. Animals with normal levels of STAT1 did not develop teratomas, nor did animals deficient in STAT1 that were of C57BL6 background that did not overexpress MMTV-neu.4 Interestingly, the studies investigating BCL-2 and human chorionic gonadotropin also used mice with an FVB/N background.
Contributing Institution:
Johns Hopkins University, School of Medicine
Department of Molecular and Comparative Pathobiology
Broadway Research Building, #811
733 N. Broadway
Baltimore, MD 21205
Phone: 443-287-2953
Fax: 443-287-5628
JPC diagnosis:
Ovary: Teratoma.
JPC comment:
Luckily, this entity is distinct from the 2014 movie "Teratoma", a 15-minute comedy from Poland. Teratomas are one of the more publicized tumors seen in humans, as the discovery of teeth and hair are surprising in the non-medical population. The word "teratoma" is derived largely from its root, terato-, which is Greek (?????) for "monster." While not monstrous themselves, their description can be a large task. Because they are derived from germ cells of the endoderm, mesoderm, and endoderm, knowing the lineage of different tissues is important. Potentially helpful mnemonics for derivatives of germ layers is offered.
ENDODERM: "endernal organs", a play on "internal". These include most internal organs and their linings, such as the gastrointestinal tract, liver, pancreas, and the respiratory system.
MESODERM: Muscle, Endothelium, Spleen, Ovaries and other gonads, Ducts of genital system, Endothelium of lymphatics, Renal, Male gonads
ECTODERM (7E's): Epidermis, Epithelial linings of external orifices, Ear, eye, and nose sensory tissues, Enamel of teeth, Exocrine glands, Encephalon (CNS), Eye lens
This is by no means complete but may assist in the quick classification of tissues.
Teratomas are classified into either mature or immature, with a histologic grading criterion attached to the latter. Mature teratomas are exclusively composed of mature tissues and are most often cystic (mature cystic teratoma) but can be solid (mature solid teratoma). While most often benign, malignant transformation can occur. The immature teratoma is defined as containing variable amounts of immature (usually primitive/embryological neuroectodermal) tissues. Both a three-tier and two-tier grading schemes have been used for the immature teratoma, with good correlation between tumor grade and prognosis.9
Recent investigative efforts have focused on melanoma-associated antigen A2 (MAGEA2) and pre-mRNA processing factor 4 (PRPF4) genes in murine embryonal stem cells (mESC) and measured teratoma formation. MAGEA2 is expressed exclusively in undifferentiated, differentiating, cancer cells, and embryonal stem cells. Early evidence suggests that increased MAGEA2 expression is associated with increased proliferative and differentiation ability of stem cells. In knockout mice, teratomas were smaller and had fewer differentiated tissues within tumors.12 Similarly, expression of PRPF4 decreased with further tissue differentiation, and knockout mouse models resulted in decreased mESC pluripotency, but increased abnormal proliferation.13 These studies, and similar studies, illustrate the complexity of embryologic tissue differentiation and our current limited understanding of the signals required for normal maturation.
The moderator mentioned that in the human literature, teratomas are starting to be classified as prepubertal or postpubertal, with prepubertal having more well differentiated tissues.
References:
1. Agnew DW, MacLachlan NJ. Tumors of the Genital System. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, Iowa: John Wiley & Sons, Inc.; 2017:698.
2. Colledge WH, Carlton MB, Udy GB, Evans MJ. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370(6484):65-68. doi:10.1038/370065a0
3. Foster RA. Female Reproductive System and Mammae. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, Missouri: Elsevier; 2017:1161.
4. Hannesdóttir L, Daschil N, Philipp S, et al. MMTV-neu mice deficient in STAT1 are susceptible to develop ovarian teratomas. Int J Dev Biol. 2012;56(4):279-283. doi:10.1387/ijdb.113397lh
5. Heaney JD, Anderson EL, Michelson MV, et al. Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development. 2012;139(9):1577-1586. doi:10.1242/dev.076851
6. Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996;137(11):4837-4843. doi:10.1210/endo.137.11.8895354
7. Huang P, Duda DG, Jain RK, Fukumura D. Histopathologic findings and establishment of novel tumor lines from spontaneous tumors in FVB/N mice. Comp Med. 2008;58(3):253-263.
8. Huhtaniemi I, Rulli S, Ahtiainen P, Poutanen M. Multiple sites of tumorigenesis in transgenic mice overproducing hCG. Mol Cell Endocrinol. 2005;234(1-2):117-126. doi:10.1016/j.mce.2004.10.013
9. Kurman RJ, Carcangiu ML, Herrington CS, Young RH, eds. Tumours of the ovary. In: WHO Classification of Tumours of Female Reproductive Organs. Lyon, France: International Agency for Research on Cancer. 2014.
10. Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging FVB/N mice. Toxicol Pathol. 1996;24(6):710-716. doi:10.1177/019262339602400606
11. McEntee K. Ovarian Neoplasms. In: Reproductive Pathology of Domestic Animals. San Diego, CA: Academic Press, Inc.; 1990:69-93.
12. Park S, Han JE, Kim HG, et al. Inhibition of MAGEA2 regulates pluripotency, proliferation, apoptosis, and differentiation in mouse embryonic stem cells. Journal of Cellular Biochemistry. 2020;121(11):4667-4679.
13. Park S, Han SH, Kim HG. Suppression of PRPF4 regulates pluripotency, proliferation, and differentiation in mouse embryonic stem cells. Cell Biochemistry and Function. 2019;37(8):608-617.
14. Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11(1):37-49. doi:10.1038/nrm2815
15. Taketo M, Schroeder AC, Mobraaten LE, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci USA. 1991;88(6):2065-2069. doi:10.1073/pnas.88.6.2065