Signalment:
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
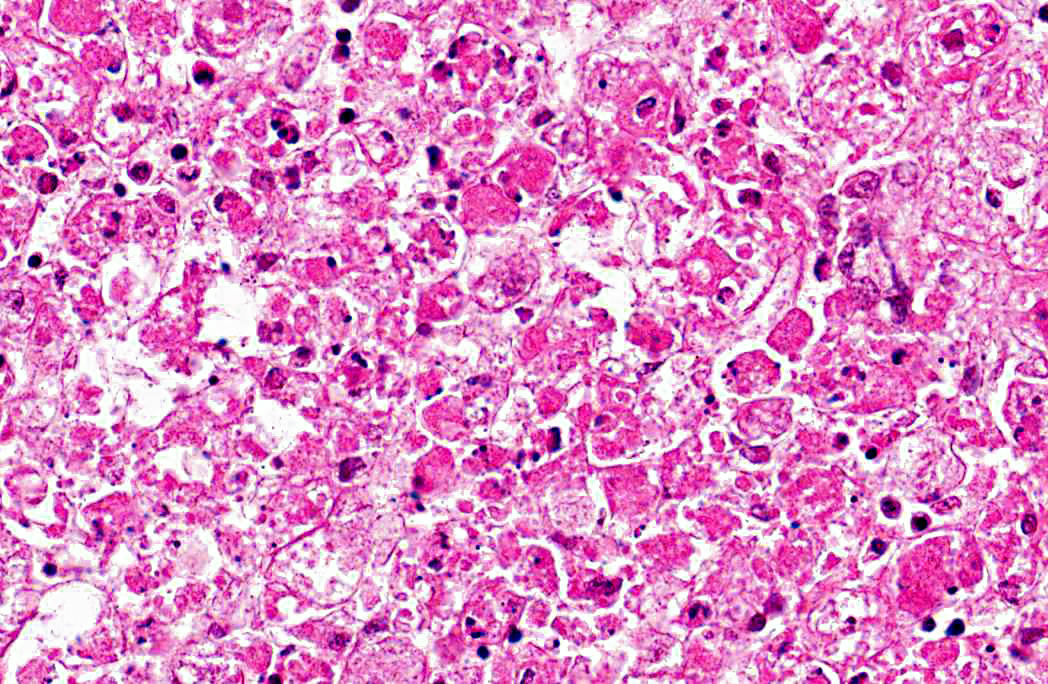
Liver: 1. Hepatocyte necrosis, peracute, diffuse (massive), severe, consistent with a diagnosis of viral haemorrhagic disease of rabbits.
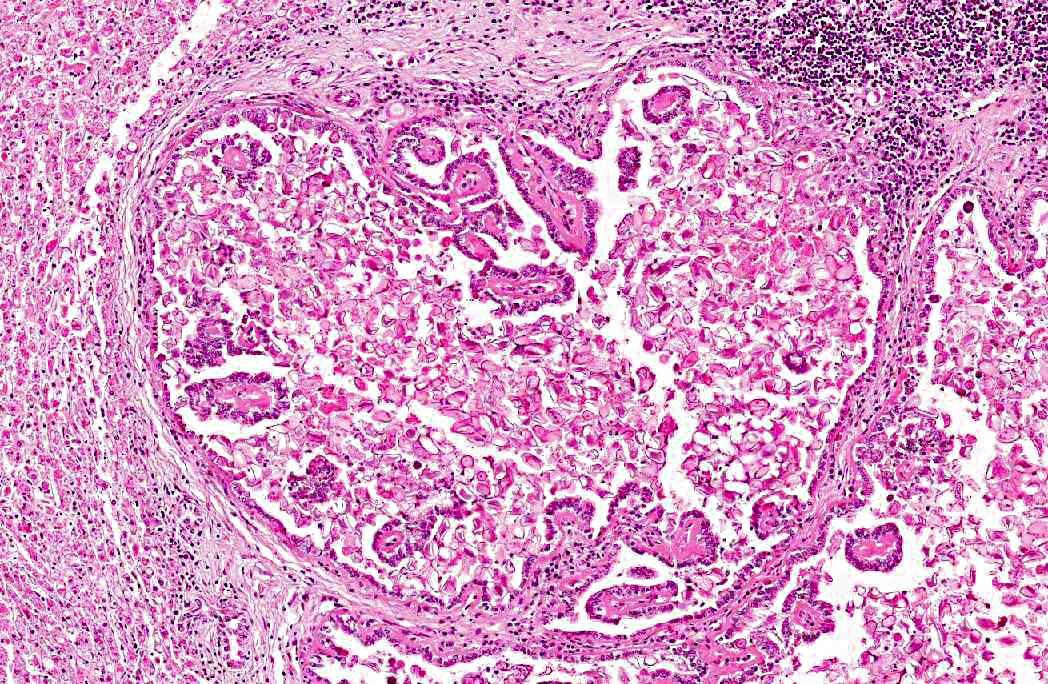
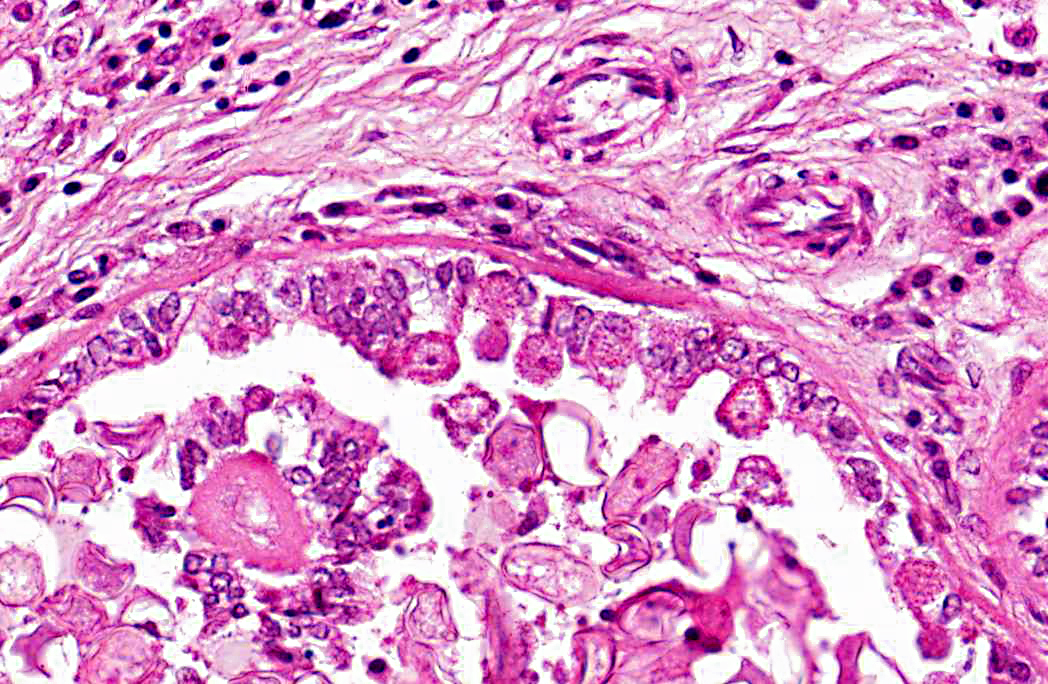
2. Bile duct hyperplasia and granulomatous cholangitis, chronic, severe with intra-luminal oocysts/macrogametes consistent with a diagnosis of hepatic coccidiosis.
Condition:
Contributor Comment:
VHD is an acute, highly fatal disease of European wild and domestic rabbits, first reported from the Peoples Republic of China in 1984.(5,10) The causative agent is a calicivirus.(10,13) The virus spreads via oral, nasal or parenteral transmission and rapidly replicates in the liver of adult rabbits resulting in death within 48 - 72 hours.(9,13) Virus-induced hepatocyte death is due to apoptosis and in experimentally induced disease, in-situ hybridization identified viral replication in both hepatocytes and macrophages, suggesting infected macrophages contribute to viral dissemination.(2,7,14) Naturally occurring VHD is rarely seen in rabbits less than two months of age, possibly due to differences in the leucocyte response to hepatocyte infection between adults and juveniles. The lymphocytic rather than heterophilic response observed in the more resistant younger rabbits possibly reflects a protective host response to viral antigens on the hepatocyte surface.(4)
Hepatic coccidiosis in rabbits occurs worldwide and is caused by infection with Eimeria stiedae.(6,16) The coccidial oocysts are highly resistant and remain viable in soil and on fomites for long periods. Rabbits become infected by ingesting sporulated oocysts which are broken down in the duodenum, releasing sporozoites that penetrate the intestinal mucosa and travel to the liver either via the blood stream or within macrophages in the lymphatic system. In the liver the sporozoites invade the bile duct epithelium. Following developmental and reproductive phases oocysts are produced and are passed via the bile ducts into the intestines. Oocysts become infective one to four days after they are shed in the faces.(6,16) The environment can become heavily contaminated in intensive conditions and wild rabbits can be a potential source of infection to domestic rabbits allowed access to grass grazed by wild rabbits.(6)
Infection of bile duct epithelial cells results in hyperplasia and inflammation with large numbers of ellipsoid oocysts in the walls and lumen of the bile ducts.(12) Destruction and regeneration of the bile duct epithelium results in significant cystic enlargement, papillomatous hyperplasia and duct reduplication. There is usually accompanying infiltration of plasma cells, lymphocytes, and occasional epithelioid cells.(12,15) Some enlarged bile ducts rupture initiating a granulomatous response and accompanying portal fibrosis is often prominent.(12) The oocysts may obstruct biliary outflow resulting in a distended bile duct and ischaemic necrosis can occur in the surrounding liver parenchyma due to compression of adjacent blood vessels by the bile duct enlargement.(12) In rabbits which survive such infection, fibrous tissue can replace much of the normal liver parenchyma.(16)
While there may be no clinical signs in mild infections, heavy infections can result in abdominal enlargement and ascites, with jaundice occurring in advanced stages of the disease. These signs reflect blockage of the bile ducts and interference with hepatic function.(12) Serum biochemistry may reveal elevated alkaline phosphatase, ALT and total bilirubin.(6)
JPC Diagnosis:
1. Liver: Necrosis, massive, diffuse.
2. Liver: Cholangiohepatitis, proliferative and lymphoplasmacytic, diffuse, moderate, with coccidial oocysts and gametocytes.
Conference Comment:
Caliciviruses are non-enveloped, icosahedral, single stranded RNA viruses with characteristic 32 cup-shaped depressions on their surface. Other caliciviruses of veterinary importance include: vesicular exanthema of swine virus, San Miguel sea lion virus, feline calicivirus, and murine norovirus. Other caliciviruses in the genera Norovirus and Sapovirus have been reported to cause disease in cattle, pigs, wildlife, and non-human primates.(8)
References:
2. Alonso C, Oviedo JM, Mart+â-¡n-Alonso JM, D+â-¡az E, Boga JA, Parra F. Programmed cell death in the pathogenesis of rabbit hemorrhagic disease. Arch Virol. 1998;143:321332.
3. Bergin IL, Wise AG, Bolin SR, Mullaney TP, Kiupel M, Maes RK. Novel Calicivirus identified in rabbits, Michigan USA. Emerg Infect Dis. 2009;15(12):1955-1962.
4. Ferreira PG, Costa-E-Silva A, Oliveira MJR, Monteiro E, Aguas AP. Leukocytehepatocyte interaction in calicivirus infection: Differences between rabbits that are resistant or susceptible to rabbit haemorrhagic disease (RHD). Vet Immunol Immunopath. 2005;103:217221.
5. Fuchs A, Weissenbock H. Comparative histopathological study of rabbit haemorrhagic disease (RHD) and European brown hare syndrome. J Comp Path. 1992;107:103-113.
6. Harcourt-Brown F. Textbook of Rabbit Medicine. Oxford, UK: Reed Educational and Professional Publishing Ltd; 2002.
7. Kimura T, Mitsui I, Okada Y, Furuya T, Ochiai K, Umemura T, et al. Distribution of rabbit haemorrhagic disease virus RNA in experimentally infected rabbits. J Comp Path. 2001;124:134-141.
8. MacLachlan NJ, Dubovi EJ. Caliciviridae. In: MacLachlan NJ, Dubovi EJ, eds. Fenners Veterinary Virology. 4th ed. San Diego, CA: Elsevier; 2011:443-450.
9. Mitro S, Krauss H. Rabbit hemorrhagic disease: A review with special reference to its epizootiology. Eur J Epid. 1993;9:7078.
10. Moussa A, Chasey D, Lavazza A, Capucci L, +à-mid B, Meyers G, et al. Haemorrhagic disease of lagomorphs: Evidence for a calicivirus. Vet Microbiol. 1992;33:375-381.
11. Ohlinger VF, Haas B, Meyers G, Weiland F, Thiel HJ. Identification of the virus causing rabbit hemorrhagic disease. J Virol. 1990;64:33313336.
12. Pakes SP, Gerrity LW. Protozoal diseases. In: Manning PJ, Ringler DH, Newcomer CE, eds. The Biology of the Laboratory Rabbit. 2nd ed. London, UK: Academic Press Limited; 1994;205-230.
13. Parra F, Prieto M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J Virol. 1990;64:40134015.
14. Prieto JM, Fernandez F, Alvarez V, Espi A, Garcia-Marin JF, Alvarez M, et al. Immunohistochemical localisation of rabbit haemorrhagic disease virus vp-60 antigen in early infection of young and adult rabbits. Res Vet Sci. 2000;68:181187.
15. Schoeb TR, Cartner SC, Baker RA, Gerrity LW. Parasites of rabbits. In: Baker DG, ed. Flynn's Parasites of Laboratory Animals. 2nd ed. Ames, Iowa: Blackwell Publishing; 2007:451-499.
16. Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. London, UK: Bailliere Tyndall; 1982.
17. Ueda K, Park JH, Ochiae K, Itakura C. Disseminated intravascular coagulation (DIC) in rabbit haemorrhagic disease. Jap J Vet Res. 1992;40:133141.