WSC 22-23
Conference 6
CASE I:
Signalment:
2-week-old, male castrated, Angus calf (Bos taurus)
History:
Ocular opacity, ocular and nasal discharge, foam coming out mouth and nose, dehydration, abdominal distention, dyspnea.
Gross Pathology:
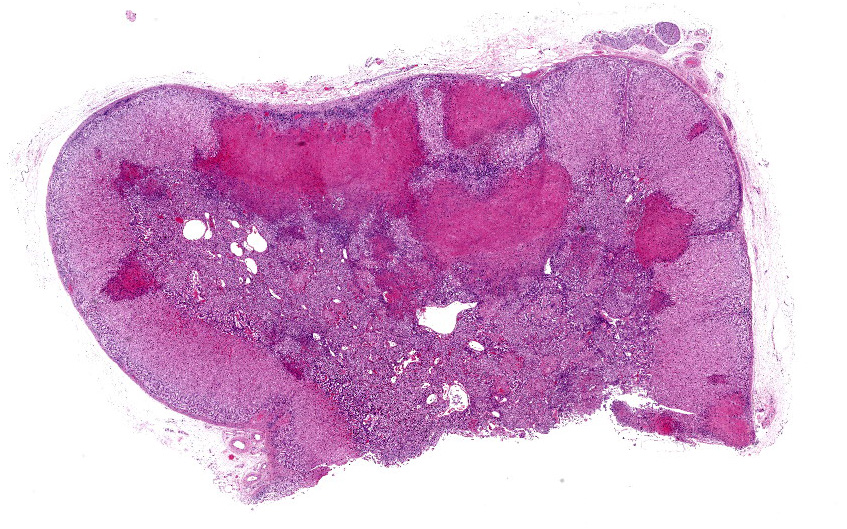
The body is in fair nutritional and postmortem condition. Multiple joints contain small amounts of fibrin. Across the mucosal surface of the larynx and trachea, there are loosely adherent, multifocal to coalescing, slightly raised, moist, tan plaques. The lungs are diffusely dark, wet, and slightly firm. Randomly distributed throughout all lung lobes are 50-100 targetoid, ~0.2 cm diameter nodules that range from tan to dark red. The myocardium heart is slightly mottled and pale. There is mild splenic enlargement and congestion of the renal medullary regions/vessels. The umbilicus is markedly thickened by fibrous tissue (chronic omphalitis). Bilaterally, the umbilical artery lumens persist and are mildly distended with opaque, brown, slightly viscous fluid (interpreted as omphaloateritis).
Laboratory Results:
PCR Results: Lung tissue tested:
Bovine coronavirus - negative
Bovine herpes virus 1 - positive (CT value = 21.77)
Bovine parainfluenza 3 - negative
Bovine syncytial virus - negative
Bovine Viral Diarrhea - negative
Influenza D virus - negative
Malignant Catarrhal Fever - negative
Mycoplasma bovis - negative
Immunohistochemistry Results: Adrenal gland:
BHV-1- positive staining (within foci of necrosis)
Aerobic Culture Results: Lung and liver:
1+ Pseudomonas aeruginosa, Staphylococcus sciuri and E. coli (postmortem overgrowth)
Microscopic Description:
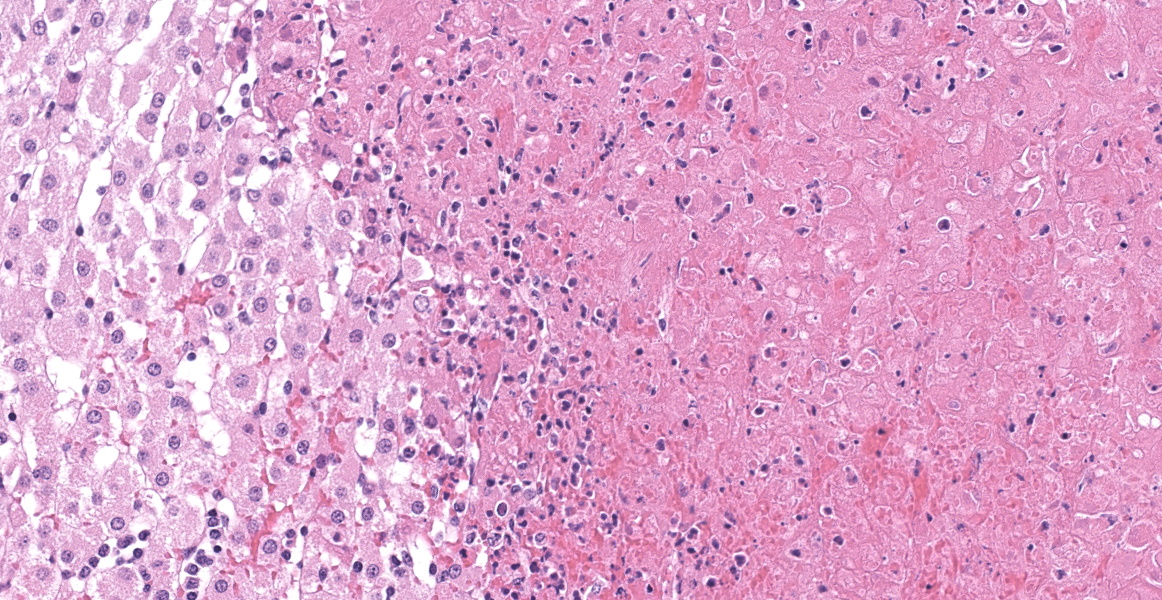
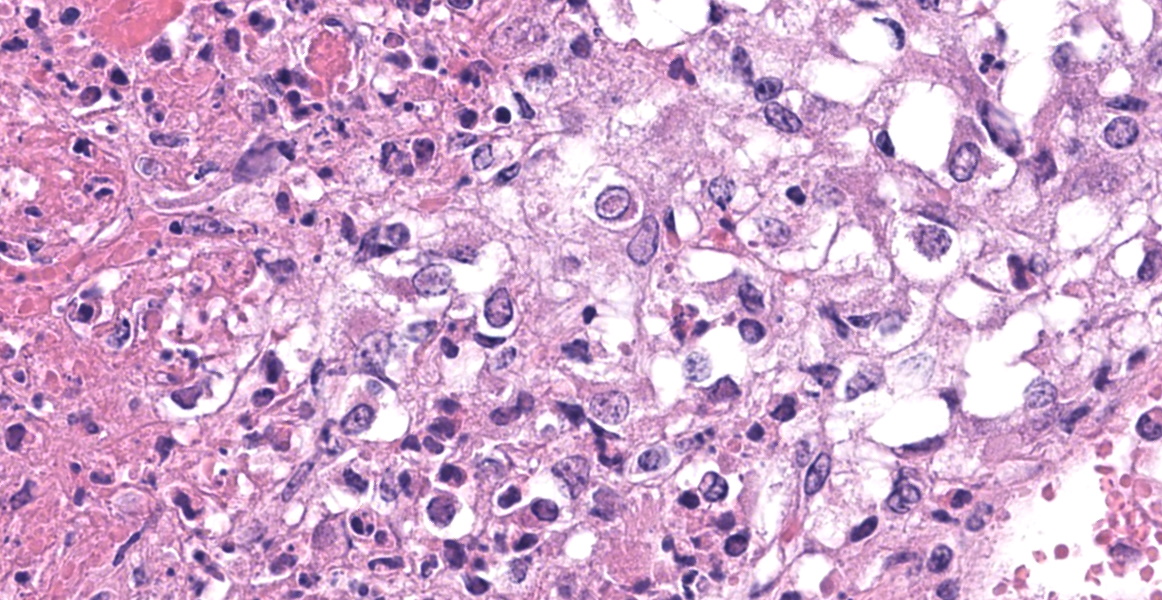
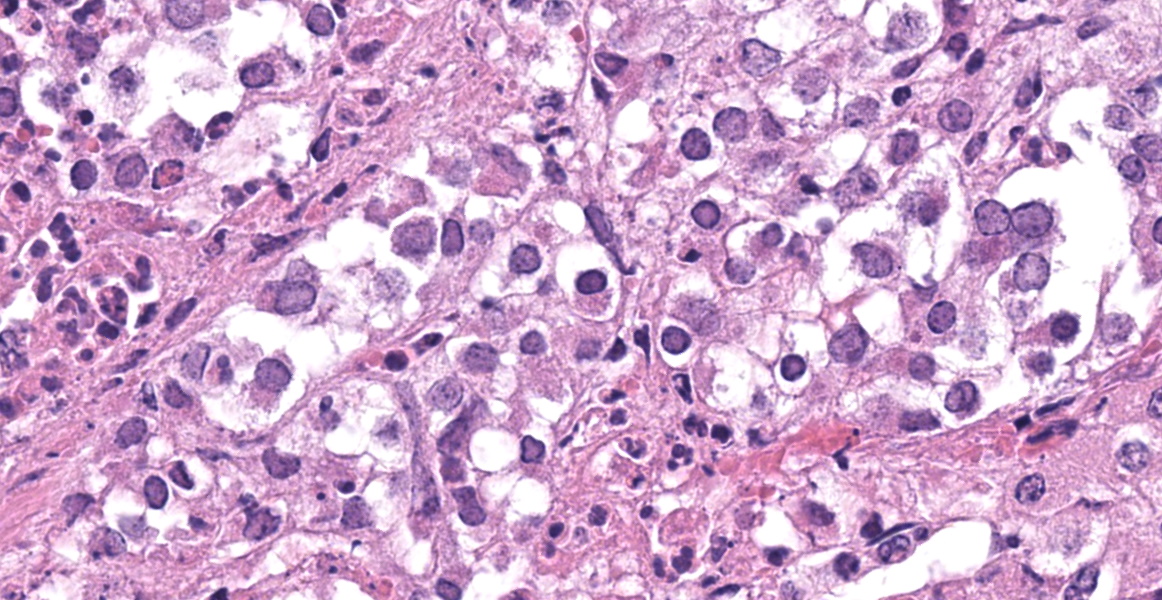
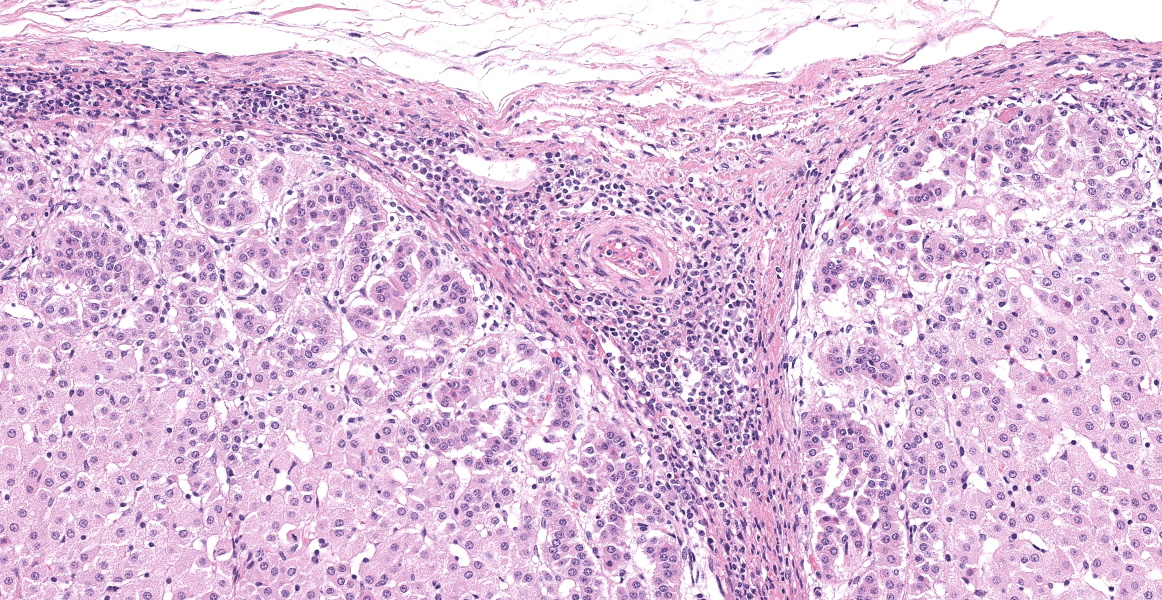
Adrenal gland: Scattered throughout the section, affecting both cortical and medullary regions, there are multifocal, random, variably sized, discrete areas of acute lytic necrosis affecting approximately 40% of the examined section. The foci of necrosis and inflammation are characterized by hypereosinophilic accumulations of necrotic cellular debris, acute hemorrhage, fibrin exudation, and infiltration by degenerating neutrophils and fewer mononuclear cells. Intact adrenal cortical and medullary cells bordering the foci necrosis frequently show marginalization of nuclear chromatin and variably frequent, 2-4 µm diameter, eosinophilic to amphophilic intranuclear inclusion bodies that are either surrounded by a clear halo or fill the nucleus. Infrequent multinucleate syncytial cells are noted at the margins of necrosis/inflammation. Similar mixtures of degenerate neutrophils and fewer mononuclear cells surround and variably infiltrate the adrenal capsule as well as associated vessels with nerves and ganglia being less affected. Subtle degeneration and lipid-vacuole loss is noted in the surrounding adipose tissue.
Immunohistochemical staining for bovine herpesvirus 1 reveals abundant positive dark brown cytoplasmic and membrane staining associated with the foci of necrosis and virus-infected cells.
Contributor’s Morphologic Diagnoses:
Adrenal gland: Adrenalitis, necrotizing, random, acute with eosinophilic intranuclear inclusion bodies (consistent with bovine herpesvirus 1 adrenalitis)
Contributor’s Comment:
Bovine herpesvirus 1 (BHV-1; Alphaherpesvirinae) infects a wide range of animals, including cattle, sheep, goats, llamas, swine, water buffalo, mustelids, and rabbits.2 The virus is widespread among cattle populations and is the cause of the following clinical disease patterns: infectious bovine rhinotracheitis (IBR), keratoconjunctivitis, bronchointerstitial pneumonia, abortion, encephalitis, systemic herpesvirus infection in young calves, and pustular vulvovaginitis or balanoposthitis in unvaccinated/naive cattle.2, 4 Routine vaccination has reduced the incidence of BHV-1 infections in both dairy and beef animals but stress, or steroid treatment, may result in reactivation of latent virus infections.4
Respiratory infections usually involve the upper respiratory tract (nasal mucosa and muzzle inflamed) and lungs and can vary from subclinical to severe infections. Young animals may also develop keratoconjunctivitis with or without upper respiratory disease, while young adult animals may also develop pustular vulvovaginitis or balanoposthitis when infected BHV-1.4
Disseminated/systemic infections with multiple organ involvement have also been identified and well documented in neonatal calves and fetuses infected with BHV-1.1, 5, 8, 14, 11 Lesions in affected neonates and fetuses have been reported to involve the upper respiratory tract, lungs, oral cavity, esophagus, rumen, liver, kidney, spleen, as well as adrenal gland,1, 5, 8, 14 with enteritis and encephalitis being observed in some clinical cases.1, 5, 14 In this case, the calf age and lesion distribution best fits with the systemic form of BHV-1 infection in young calves.
The adrenal lesions are consistent with those previously reported in systemic herpesvirus infection and are characterized as multifocal random foci of necrosis range from coagulative to lytic) accompanied by infiltration with scattered degenerate neutrophils.1,12 Like other reports, occasional eosinophilic intranuclear viral inclusion bodies and syncytial cells are observed amongst the necrotic cells as well as within intact virus-infected cells bordering foci of necrosis.1, 2, 12 As Moeller Jr. and others (2013) noted, microscopic lesions associated with neonatal BHV-1 infection may be confined to the adrenal gland only (29/62 animals examined had adrenal lesions only) and the diagnosis may be missed if adrenal gland is not sampled during postmortem examination.12
The source of BHV-1 transmission from infected dams to calves may occur in utero (late gestation) or during parturition and can result in fetal abortion or systemic disease in neonatal or suckling calves (up to 1 month of age; clinical disease following a 2-6-day incubation period).2, 11, 12 Reactivation of latent infections or activation of modified live vaccine strain virus may also be linked to environmental stressors and/or immunosuppression (e.g., inadequate colostrum intact) have been shown to contribute to the expression of clinical disease in calves and cattle infected BHV-1.1, 9, 10 A limited vaccination history of dams and potential inadequate colostrum intact may account for the development of systemic disease in this calf.
Contributing Institution:
Department of Veterinary Clinical and Diagnostic Sciences with the Faculty of Veterinary Medicine, University of Calgary.
JPCDiagnosis:
Adrenal gland: Adrenalitis, necrohemorrhagic, multifocal to coalescing, moderate, with intranuclear viral inclusions and viral syncytia.
JPC Comment:
Bovine herpesvirus-1 is a double-stranded DNA virus which was first isolated in 1956.3 There are three subtypes, BHV 1.1, 1.2a, and 1.2b, which have slight variations in clinical presentation, pathogenicity, and geographic distribution.6 All subtypes can cause infectious bovine rhinotracheitis which is the most common presentation in feedlot cattle. BHV-1 is similar to Mycoplasma bovis, as seen in Conference 1 Case 3 earlier this year, in that it can damage host respiratory defense mechanisms and predispose to secondary bacterial invaders, culminating in bovine respiratory disease complex (shipping fever), an economically important disease which costs at least $1 billion annually in the United States alone.7
BHV lacks tissue tropism and can infect a wide range of cells, as illustrated in this case of systemic disease. BHV-1 attaches to the cell surface using envelope proteins gB and gC, interacts with the intercellular adhesion molecule Nectin-1 using envelope protein gD, and enters the cell after viral and cell membrane fusion.6 The virus can spread directly between cells without cell lysis using a variety of other glycoproteins and can spread systemically through a cell-associated viremia.3,6,13 BHV-1 can also spread through rupture of infected cells. The virus induces production of “virus host shut off” (vhs) protein, so named because it disables cellular protein synthesis, compromises membrane stability, and ultimately results in cell necrosis.3 Virus particles liberated from ruptured cells can subsequently infect adjacent cells.3 In the airways, necrosis of epithelial cells disrupts the mucocilliary apparatus, preventing clearance of inhaled particles and allowing bacterial deposition within the lungs.3 Additional, the virus can replicate in endothelial cells, causing vascular damage and ischemia. Conference participants remarked on the lack of viral inclusions in the endothelium in this case, which was surprising given the degree of necrosis in the section.
BHV-1 also induces a transient immunosuppression through a variety of mechanisms. Viral infection induces apoptosis of CD4+ T cells and impairs CD8+ CTL function by inhibiting antigen processing and repressing MCHI expression.6 The vhs protein inhibits MHCII expression. Finally, viral bICP0 gene activity inhibits interferon gamma production by inhibiting interferon regulatory factor (IRF) 7 and stimulating proteosomic descruction of IRF3.6
Latency and recrudescence are a hallmark of herpesviral infection and make control and eradication of BHV-1 difficult.3,13 After initial infection, BHV-1 spreads between cells and ultimately enters the peripheral nervous system.3 In respiratory infections, the virus becomes latent in the trigeminal ganglion, while genital infections result in latency in the sciatic nerve.13 Latency in other organs, including the tonsils, lymph nodes, blood, and spleen, has also been documented.6 Latent infection and recrudescence are controlled by a few viral proteins and the host immune system.6 The latency-related (LR) gene is active early in infection of neurons and helps inhibits neuronal apoptosis, productive infection, and bICP0 expression.6 Active cell-mediated immunity also enforces latency, and CD8+ T cells which persist in the infected ganglia produce IFN-gamma and may induce cytotoxicity to reduce viral spread.6 Stress (i.e. from shipping or introduction into a new herd) or administration of corticosteroids is associated with reduced LR expression, active viral replication, and recrudescence. 6
References:
- Bryan LA, Fenton RA, Misra V, Haines DM. Fatal, generalized bovine herpesvirus type-1 infection associated with a modified-live infectious bovine rhinotracheitis parainfluenza-3 vaccine administered to neonatal calves. Can Vet J. 1994;35: 223-228.
- Caswell JL, Williams KJ. Chapter 5 - Respiratory System. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 2. 6th Philadelphia, PA: Elsevier Saunders; 2016:465-591, e464.
- Ellis JA. Update on viral pathogenesis in BRD. Animal Health Research Reviews. 2010; 10(2):149-153.
- Graham DA. Bovine herpes virus-1 (BoHV-1) in cattle-a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom. Ir Vet J. 2013;66: 15.
- Hill BD, Hill MW, Chung YS, Whittle RJ. Meningoencephalitis in calves due to bovine herpesvirus type 1 infection. Aust Vet J. 1984;61: 242-243.
- Jones C, Chowdhury S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Animal Health Research Reviews. 2008; 8(2):187-205.
- Jones C, Chowdhury. Bovine Herpesvirus Type 1 (BHV-1) is an Important cofactor in the Bovine Respiratory Disease Complex. Vet Clin Food Anim. 2010; 26:303-321.
- Kennedy PC, Richards WPC. The Pathology of Abortion Caused by the Virus of Infectious Bovine Rhinotracheitis. Pathologia veterinaria. 1964;1: 7-17.
- Mechor GD, Rousseaux CG, Radostits OM, Babiuk LA, Petrie L. Protection of newborn calves against fatal multisystemic infectious bovine rhinotracheitis by feeding colostrum from vaccinated cows. Can J Vet Res. 1987;51: 452-459.
- Meyer G, Lemaire M, Ros C, et al. Comparative pathogenesis of acute and latent infections of calves with bovine herpesvirus types 1 and 5. Arch Virol. 2001;146: 633-652.
- Miller RB, Smith MW, Lawson KF. Some lesions observed in calves born to cows exposed to the virus of infectious bovine rhinotracheitis in the last trimester of gestation. Can J Comp Med. 1978;42: 438-445.
- Moeller RB, Adaska J, Reynolds J, Blanchard PC. Systemic Bovine herpesvirus 1 infections in neonatal dairy calves. Jour Vet Diag Invest. 2013;25: 136-141.
- Osterrieder K. Herpesvirales. In: MacLachlan NJ, Dubovi EJ, eds. Fenner’s Veterinary Virology. 5th ed. Cambridge, MA: Elsevier. 2017; 194-195, 199-201.
- Penny CD, Sargison ND, Howie F, Nettleton PF, Schock A. Upper respiratory disease and encephalitis in neonatal beef calves caused by bovine herpesvirus type 1. Veterinary Record. 2002;151: 89-91.