WSC 22-23
Conference 9
Case IV
Signalment:
9-year-old, male castrated, Shepherd Mix dog (Canis lupus familiaris)
History:
Presented to the hospital with a history of lethargy, decreased appetite, unwilling to walk and vomiting. The patient was alert, responsive and with cold distal extremities. On physical examination, the temperature was 97.0 F, heart rate was 76 beats/min and respiratory rate was 56 breaths/min. Left ventricle not contracting appropriately and an enlarged left adrenal gland extending into the phrenicoabdominal vein were identified on ultrasound. In addition, the patient had muffled heart sounds and weak femoral pulses.
Gross Pathology:
The right atrium and the right ventricle had multiple linear, flat dark red 2-cm to 4-cm long streaks around multiple blood vessels. The right and left free ventricular wall ratio was 1:3, and the heart weighed 472-g, which was 1.23% of the body weight (normal range in adult dog is 0.7-1.2%).
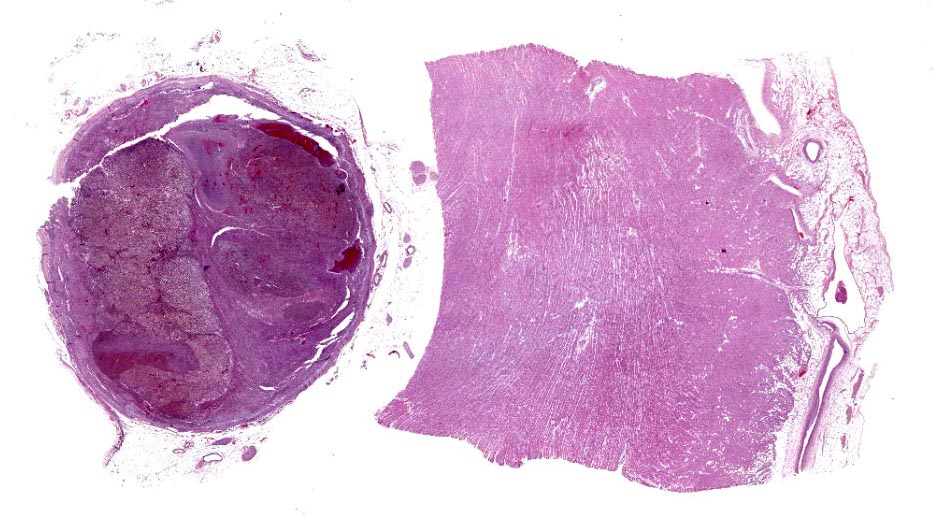
The left adrenal gland was enlarged (3.2-cm x 2.5-cm x 1.6-cm), multinodular, and firm. On cut surface, the corticomedullary ratio was 1:10 with a red to dark red, 2.5-cm in diameter mass expanding and replacing the medullary and markedly compressing the cortex. This adrenal gland was firmly attached and penetrated to the adjacent caudal vena cava. The affected caudal vena cava was segmentally and extensively dilated and completely occluded by a dark red, 3.5-cm x 1.5-cm x 1.2-cm cylindrical, firm tumor embolus firmly attached to the vascular intima. The right adrenal gland was 2.5-cm x 1-cm x 0.5-cm, with a distinct corticomedullary junction, yet medulla expanded by ill-defined light brown nodules.
Laboratory Results:
None reported.
Microscopic Description:
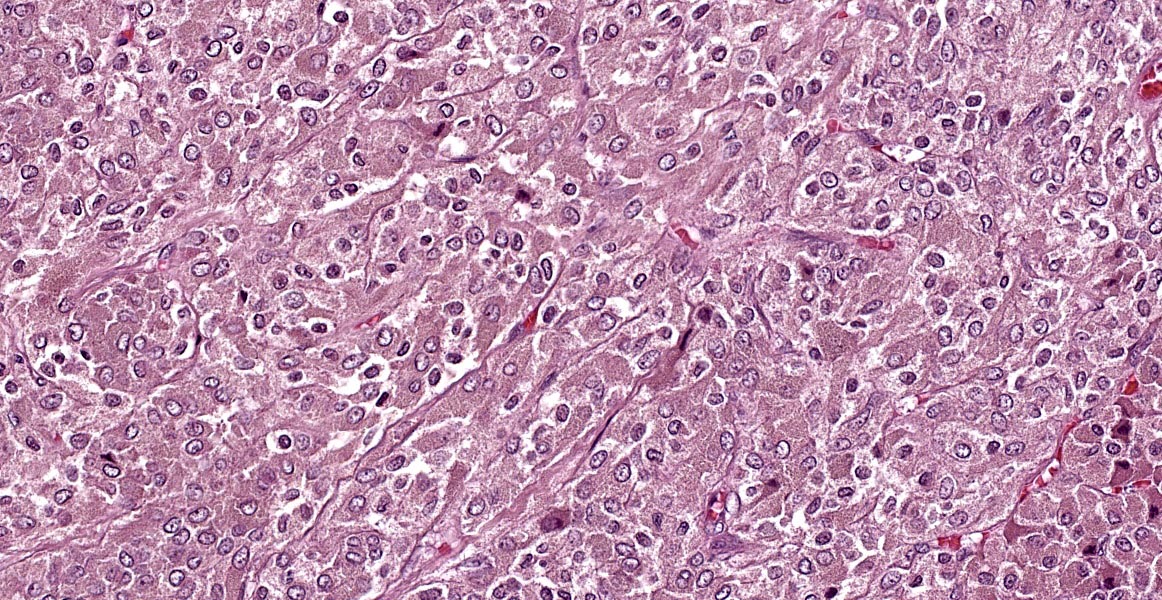
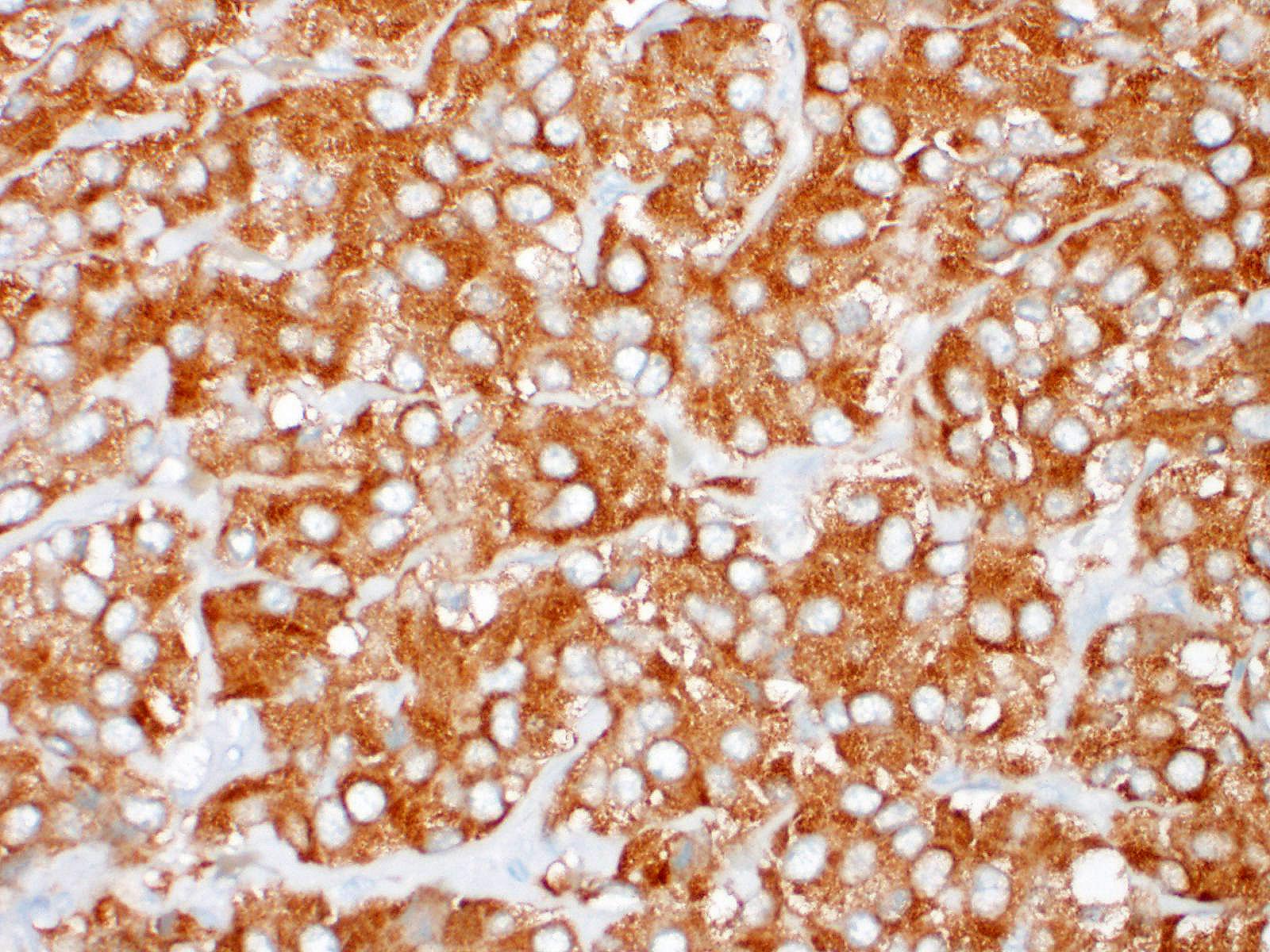
Left adrenal gland (not included in this submission’s slide): Markedly effacing and expanding the medulla and compressing the cortex is a nonencapsulated, infiltrative, highly cellular neoplasm, composed of round to polygonal cells arranged in dense sheets, nests and packets, supported by delicate fibrovascular stroma. There are extensive areas of necrosis, hemorrhage, fibrin and moderate numbers of degenerate neutrophils along with large clusters of neoplastic cells that invade the caudal vena cava wall and form an intravascular tumor cell thrombus. The neoplastic cells have distinct cell borders, moderate amounts of faintly granular cytoplasm, and a round to oval nucleus with finely stippled chromatin and 1-2 nucleoli. Anisocytosis and anisokaryosis are mild and there are 5 mitotic figures in ten 400x fields (2.37 mm-2). The associated celiac ganglion is focally infiltrated by lymphocytes and plasma cells.
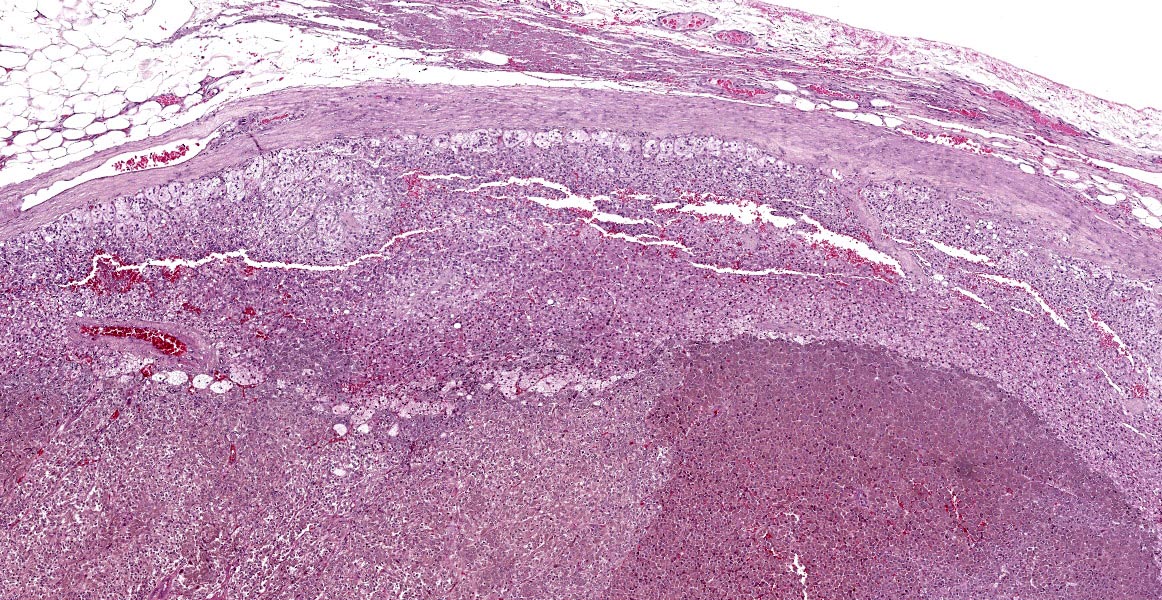
Right adrenal gland (included in this submission’s slide): Markedly expanding the medulla and compressing the cortex are multifocal to coalescing nodules comprised by neoplastic cells with similar arrangement and cytonuclear features as those described in the left adrenal gland.
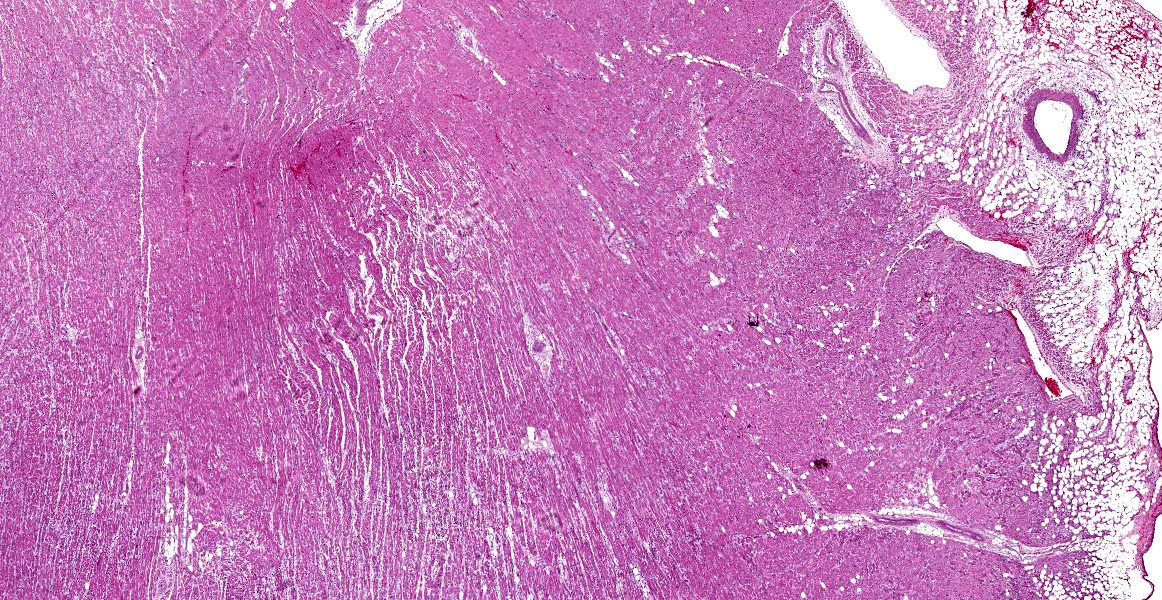
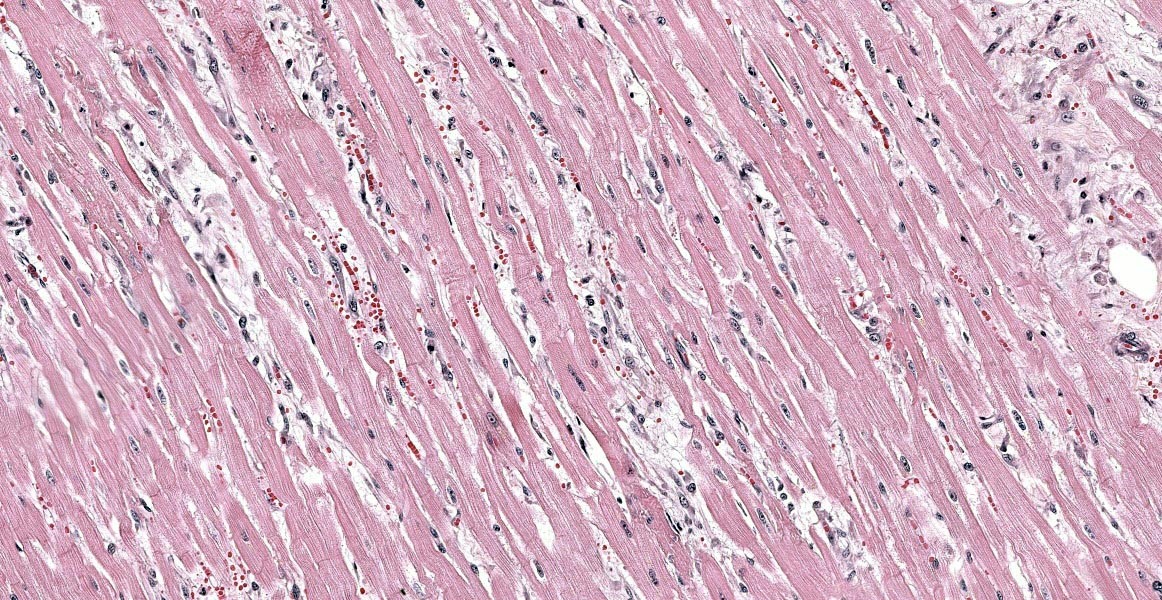
Heart: Diffusely, the myocardial interstitium of the left ventricle is mildly expanded by edema and mildly increased numbers of fibrocytes and fibroblasts. Multifocally, individualized or clusters of cardiomyocytes exhibit pale sarcoplasm (degeneration) or bright eosinophilic sarcoplasm with loss of cross striations and fragmentation, and a pyknotic or karyolitic nucleus (necrosis). Often adjacent to or surrounding these myocytes are infiltrates of histiocytes and rare lymphocytes. Within patchy regions of denser fibrosis, myocytes are separated and atrophied. The tunicas media and adventitia of the multiple epicardial branches of the coronary artery are circumferentially expanded and effaced by hyalinized eosinophilic material (fibrin), and hemorrhage accompanied by perivascular edema and infiltrates of moderate numbers of macrophages, plasma cells and nuclear debris. Occasionally, the blood vessels have plump medial smooth muscle cells and endothelium.
Contributor’s Morphologic Diagnoses:
Adrenal glands: Bilateral adrenal pheochromocytomas with caudal vena cava invasion
Heart: Moderate, multifocal, myocardial degeneration and necrosis with coronary arterial fibrinoid necrosis
Contributor’s Comment:
Pheochromocytoma is the most common neoplasm of the canine adrenal medulla, arising from chromaffin cells, which physiologically release catecholamines.3,7,10 Although pheochromocytomas are generally unilateral, they can involve both adrenal glands. These tumors can be benign or malignant, the latter associated with invasion beyond its capsule and, in some instances, to the vena cava and/or phrenicoabdomminal vein as well as adjacent vessels. Metastasis to lung, spleen, liver, heart, bone or regional lymph nodes can also occur.14 Pheochromocytomas can be nonfunctional or functional. Those tumors with functional activity can secrete catecholamines, being norepinephrine the most frequently reported.6
Catecholamines include epinephrine (adrenaline), norepinephrine (noradrenaline) and dopamine.12 These amines are synthesized from the amino acid tyrosine, which are derived from food or formed from phenylalanine in the liver. Acting as postsynaptic neurons, chromaffin cells release secretory products when triggered by nerve impulses carried by the sympathetic fibers. The secretory products then bind to the G-protein coupled receptors located on targeted organs. Two broad receptor classifications, α and β, have been described, and they can further expand into nine receptor subtypes. Both epinephrine and norepinephrine can bind and act on adrenergic α and β receptors. The catecholamines have a profound physiology effect on blood vessels and heart. Different concentration of epinephrine can elicit opposite reactions on the vasculature. At low concentration, epinephrine can lead to vasodilation by binding to β2; while at higher concentration, mainly stimulates α1 receptor and triggers vasoconstriction. In addition, increased force and rate of contraction of myocardium can occur with binding to β1 receptor.7,12 Clinical signs are often the result of excessive catecholamine secretion from the pheochromocytoma; however, the amount of secretion is variable, sporadic and unpredictable.7 Therefore, approximately half of the cases are incidental findings at necropsy or during surgery.
In human and animals, weakness, tachypnea, lethargy, anorexia, vomiting, high blood pressure or cardiac arrhythmias are the most frequently reported clinical signs in pheochromocytoma-affected patients.4,12,13 Regarding myocardial changes, the theory of pheochromocytoma-associated catecholamine cardiomyopathy has been introduced.6 However, the mechanism of the cardiomyopathy is multifactorial.3 Myocardial injuries from circulating catecholamines have been observed in several animal models. The proposed pathogenesis of myocardial damage includes excessive stimulation of adrenergic amines that induce vasoconstriction and vasospasms in the coronary artery, leading tomyocardial hypoxia and/or infarction. In addition, the aberrant concentration of catecholamines has been shown to increase permeability of the sarcolemmal membrane and lead to increased intrasarcoplasmic calcium influx that directly causes cardiomyocyte toxic damage. Moreover, the excessive catecholamine-induced systemic hypertension as well as overloaded pressure contribute to cardiomyocyte hypertrophy and secondary hypertrophic cardiomyopathy. Histologic changes include multifocal cardiomyocyte necrosis with contraction bands, cardiomyocyte degeneration, myocardial hemorrhage, myocarditis and interstitial fibrosis.6
In the present case, not only similar histologic changes are present in the cardiomyocytes and interstitium, but coronary arteries exhibit vasculitis / fibrinoid change. Mechanisms leading to vasculitis and fibrinoid change are variable, including systemic hypertension, stress-induced, idiopathic, as well as primary or secondary to inflammatory responses or immune-mediated diseases.15 No profound inflammation or immune-mediated disease were seen in this case. In addition, hyperthyroidism, diabetes mellitus and hyperaldosteronism were less likely based on clinical and pathologic examination. In cases of pheochromocytomas or hypertension, patients can have adaptive mural thickening, arteriolosclerosis or fibrinoid necrosis in heart, kidney, lung or spleen.6 It is intriguing that the vasculitis was confined to the coronary arteries in this patient, and the cause is highly suspected to be pheochromocytoma-related.
Contributing Institution:
Auburn University
166 Greene Hall
Pathobiology
College of Veterinary Medicine
Auburn, AL
36849-5519
JPC Diagnosis:
- Adrenal gland: Pheochromocytoma.
- Heart: Arteriolar fibrinoid necrosis, multifocal, moderate with myocardial degeneration, loss, and fibrosis.
JPC Comment:
The contributor provides an excellent overview of adrenal pheochromocytomas and the systemic effects of excessive catecholamine production. Pheochromocytomas occur in many veterinary species, and in clouded leopards specifically they are the most common neoplasm, occurring in 1% of animals in one study of 271 animals.16 In a separate review, pheochromocytomas accounted for 36% of reported cases of neoplasia in clouded leopards (41 of 144 cases).9
Other neoplasms which may originate from the adrenal medulla include neuroblastomas and ganglioneuromas, both of which are rare tumors of neuroectoderm origin.14 Neuroblastomas originate from primitive neuroectodermal cells and can arise anywhere in the sympathetic nervous system.1 Adrenal neuroblastomas occur in children, and are rare in dogs, ferrets, and cows.1,2 Histologically, neuroblastomas are infiltrative, densely cellular neoplasms composed of small cells resembling lymphocytes with scant cytoplasm.1,14 Occasionally, neoplastic cells form Homer-Wright rosettes and pseudorosettes.1 There is limited information on the biologic behavior of this neoplasm, but in dogs, it is likely malignant and may metastasize within the abdomen.1
Ganglioneuromas, on the other hand, are benign tumors which have been diagnosed in humans, dogs, cows, rats, and a cotton top tamarin.5,8,11 The neoplasms are composed of ganglia cells admixed with satellite cells and Schwann cells in a neurofibrillary matrix with prominent fibrous connective tissue.11,14 The ganglion cells have significant anisocytosis, abundant finely granular cytoplasm, and large round heterochromatic nuclei.11 Occasionally, ganglioneuromas occur alongside pheochromocytomas, and in one study of rats, all 9 ganglioneuromas were associated with pheochromocytomas.11
References:
- Arenas-Gamboa AM, Tanabe M, Edwards J, Storts R. Peripheral Neuroblastomas in Dogs: A Case Series. J Comp Path. 2014; 150: 361-365.
- Bakthavatchalu V, Muthupalani S, Marini RP, Fox JG. Endocrinopathy and Aging in Ferrets. Vet Pathol. 2016; 53(2): 349-365.
- Barthez PY, Marks SL, Woo J, Feldman EC, Matteucci M. Pheochromocytoma in dogs: 61 cases (1984-1995). J Vet Intern Med. 1997:11(5):272-278.
- Dagartzikas MI, Sprague K, Carter G, Tobias JD. Cerebrovascular event, dilated cardiomyopathy, and pheochromocytoma. Pediatr Emerg Care. 2002 Feb;18(1):33-5.
- Dias JLC, Montali RJ, Strandberg JD, Johnson LK, Wolff MJ. Endocrine neoplasia in New World primates. J Med Primatol. 1996; 25: 34-41.
- Edmondson EF, Bright JM, Halsey CH, Ehrhart EJ. Pathologic and cardiovascular characterization of pheochromocytoma-associated cardiomyopathy in dogs. Vet Pathol. 2015;52(2):338-343.
- Feldman E, Nelson R. Pheochromocytoma and multiple endocrine neoplasia. In: Canine and Feline Endocrinology and Reproduction. 3rd ed. Philadelphia, PA: WB Saunders;2003:440-463.
- Grossi AB. Leifsson PS. Jensen HE. Vainer B. Iburg T. Histologic and Immunohistochemical Classification of 41 Bovine Adrenal Gland Neoplasms. Vet Pathol. 2012; 534-542.
- Mathieu A, Garner MM. A Retrospective Study of Neoplasia in Non-domestic Felids in Human Care, with a Comparative Literature Review. J Zoo Wildl Med. 2021; 52(2): 413-426.
- Miller MA. Endocrine system. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th St. Louis, MO: Elsevier; 2017:709.
- Pace V, Perentes E, Germann PG. Pheochromocytomas and Ganglioneuromas in the Aging Rat: Morphological and Immunohistochemical Characterization. Tox Pathol. 2002; 30(4):492-500.
- Reusch CE. Pheochromocytoma and multiple endocrine neoplasia. In: Scott-Moncrieff, CR, ed. Canine and Feline Endocrinology. 4th Saunders; 2015: 521-551.
- Rosol TJ, Grone A. Endocrine glands. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 3. 6th ed. St. Louis, MO: Elsevier; 2016:343-344, 349-352.
- Rosol TJ, Meuten DJ. Tumors of the endocrine glands. In: Meuten DJ, ed. Tumors in Domestic Animals. Ames, IA: John Wiley and Sons, Inc.; 2017:787-791.
- Stone JR. Diseases of small and medium-sized blood vessels. In: Buja LM, ed. Cardiovascular Pathology. 4th Academic press; 2016: 125-168.
- Thorel M, Pignon C, Arne P, Donnelly TM, Riviere J. Clouded Leopard (Neofilis nebulosa) Morbidity and Mortality in Captive-Bred Populations: A Comprehensive Retrospective Study of Medical Data from 271 Individuals in European, Asian, and Australian Zoos. J Zoo Wildl Med. 2020; 51(1); 150-158.