CASE 2: 17-0367-2-3 (JPC 4101224-00)
Signalment:
Twelve-month-old, intact male Sprague-Dawley rat, Rattus norvegicus.
History:
The animal was part of a group of training rats; however, it had never been used for this purpose and was considered experimentally naïve. Health records indicated that the animal was reported clinically normal. During a recent regular check-up, a large ovoid mass was found slightly protruding from the right flank. The animal was euthanized and submitted for necropsy.
Gross Pathology:
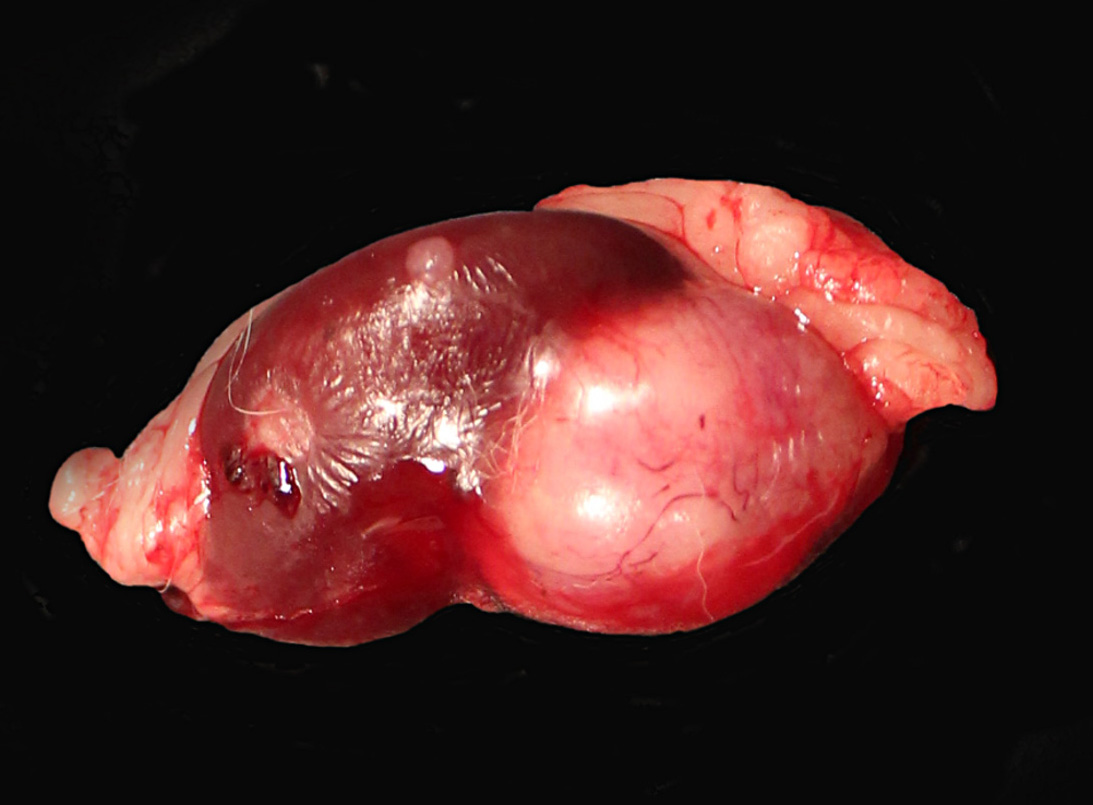
The right kidney was completely effaced by a 3 cm x 2.5 cm, white to grayish, firm multinodular mass with multifocal areas of hemorrhages and necrosis. A similar but smaller (1.2 cm in diameter) round white firm mass was also present on the caudal pole of the left kidney.
Laboratory results:
Not applicable.
Microscopic description:
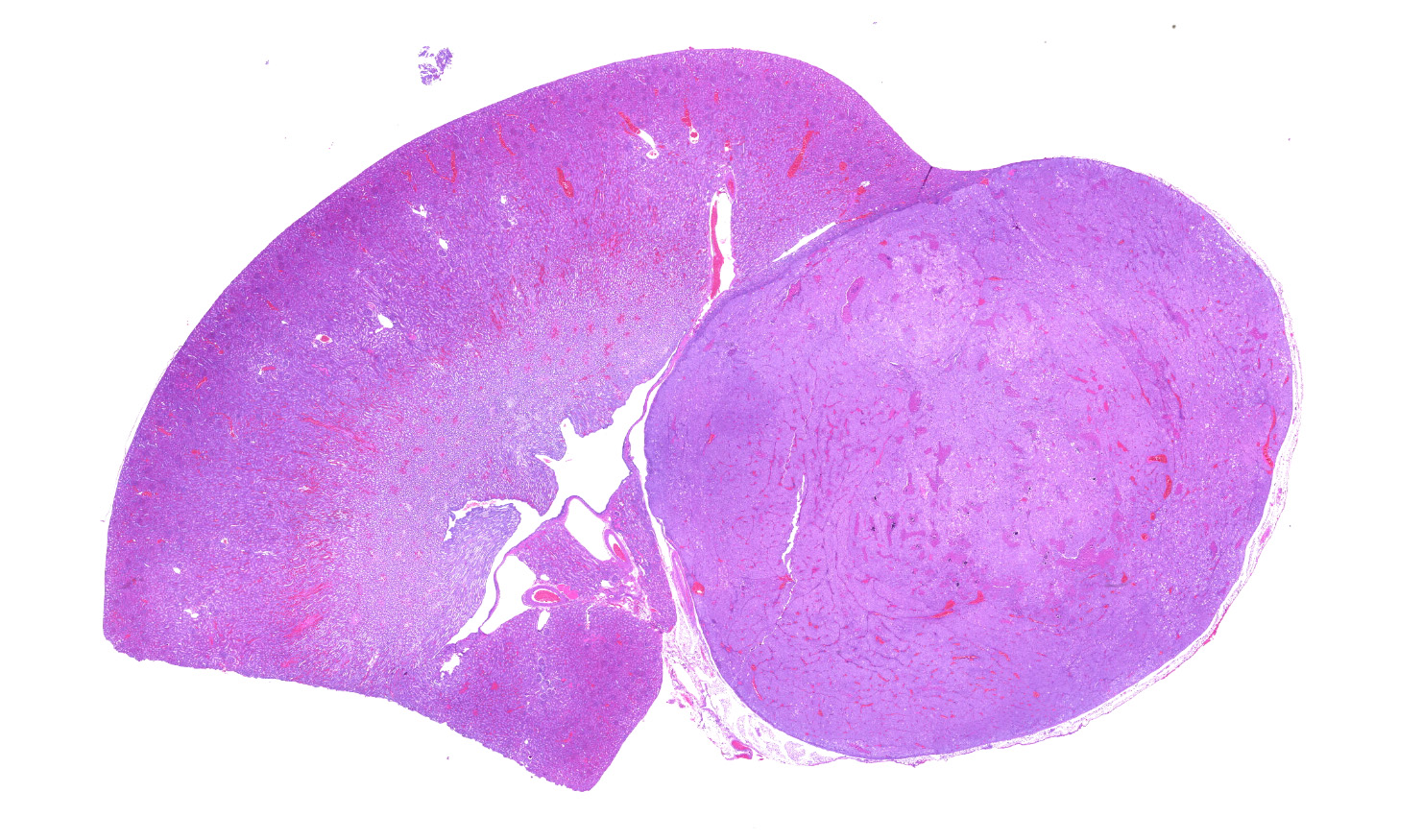
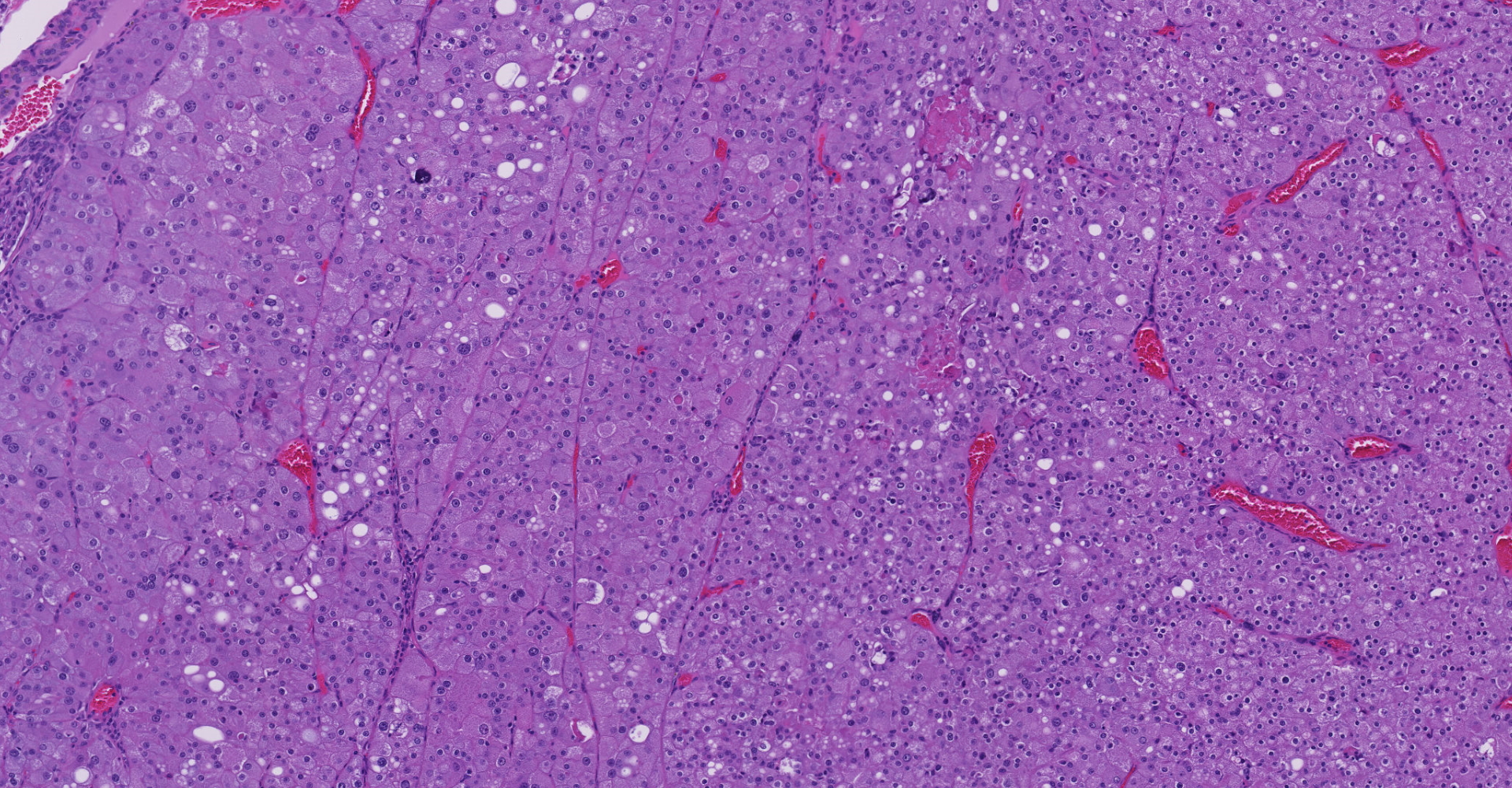
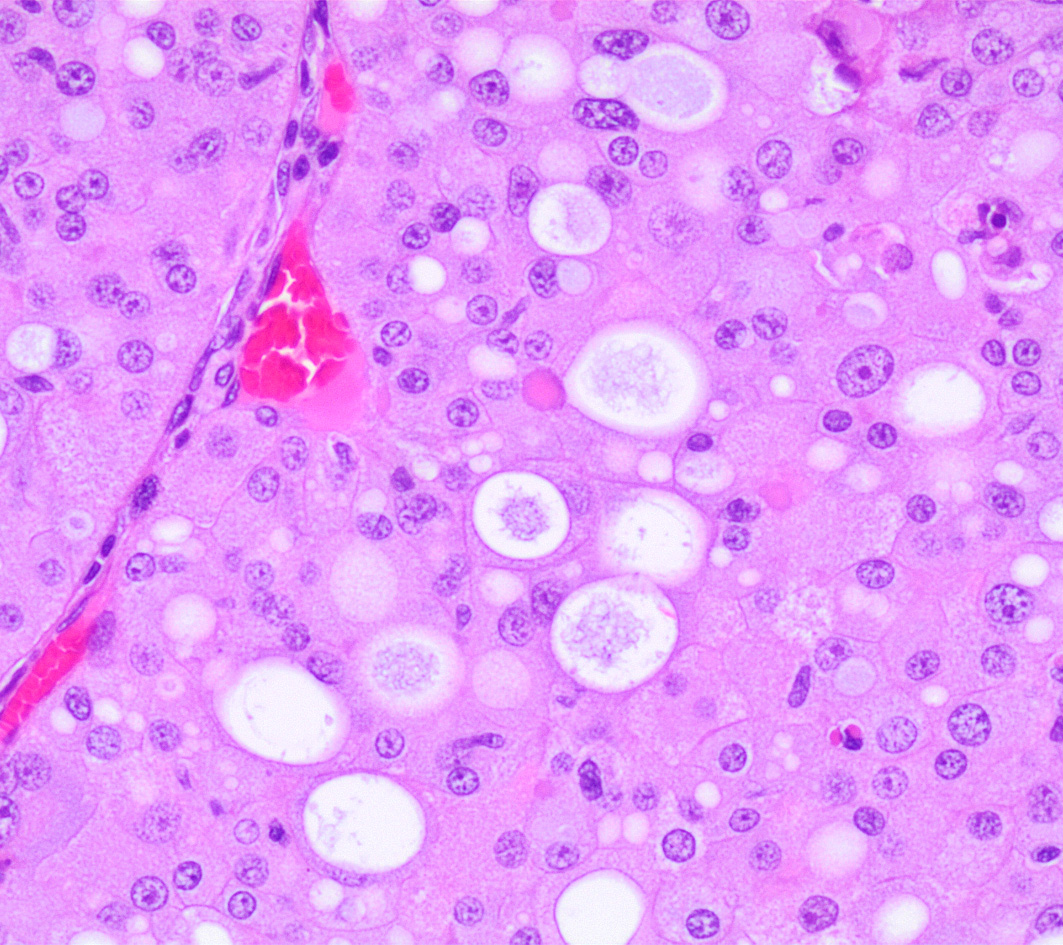
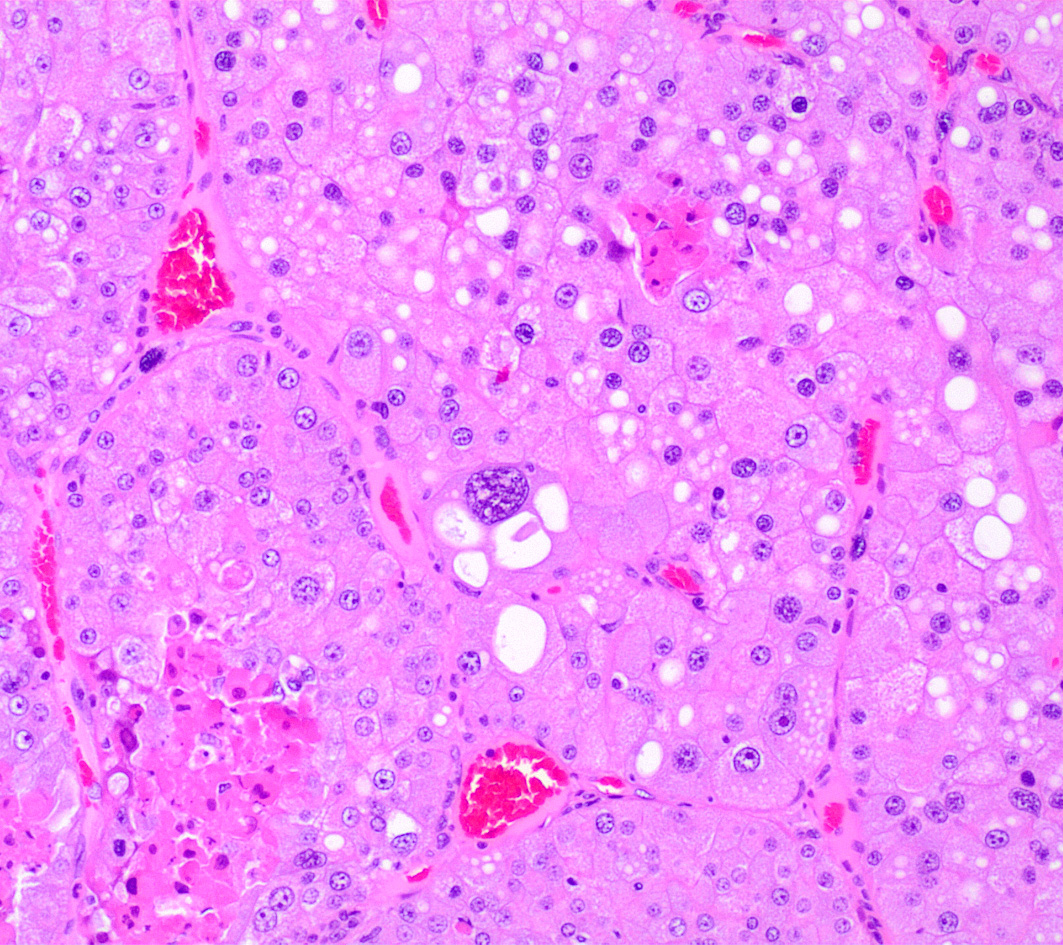
Left kidney. Partially effacing the right kidney and compressing the adjacent normal parenchyma, there is a well demarcated, unencapsulated, expansile multinodular epithelial neoplasm characterized by a diffuse lobular pattern in which lobules are separated by thin bands of fibrovascular stroma. Lobules are composed of large, round to polygonal cells, with abundant amphophilic to eosinophilic cytoplasm. Neoplastic cells frequently display multiple small clear vacuoles within their cytoplasm or contain large clear intracytoplasmic vacuoles. Nuclei are round to oval with finely granular chromatin and 1 occasionally evident central magenta nucleolus. There is moderate cellular and nuclear pleomorphism with karyomegaly, and mitoses are rare. Scattered throughout the neoplasm, there are multifocal areas of coagulative necrosis within the center of the lobules, mineral deposition is seen. In the adjacent section of preserved kidney, tubules are occasionally dilated, lined by flattened tubular epithelial cells, and contain a moderate to abundant amount of eosinophilic homogeneous proteinaceous material. Occasional tubular basophilia is seen with mild thickening of the peritubular basement membrane. Small clusters of lymphocytes and plasma cells are rarely seen in the fibrovascular stroma adjacent to the neoplastic cells of within the preserved renal interstitium.
Contributor's morphologic diagnosis:
Kidneys: Amphophilic-vacuolar renal tubule carcinoma, bilateral.
Kidney, left: Mild multifocal tubular dilation with intraluminal hyaline casts and peritubular basophilia; minimal multifocal interstitial lymphoplasmacytic infiltrates.
Contributor's comment:
Amphophilic-vacuolar (AV) tumors are a variant of renal tubule tumors (RTTs) exhibiting morphologic features that are different from the conventional RTTs.1,7,8,9 Specifically, AV tumors have a diffuse multilobular appearance in which lobules are separated by thin bands of fibrovascular stroma.1 Lobules consist of tightly packed polygonal cells with distinct cell borders, abundant amphophilic to eosinophilic cytoplasm frequently containing numerous large clear vacuoles.1,9
Tumors with AV phenotype can be seen in rats as part of the spectrum of hyperplasia, adenoma, and carcinoma identified for renal tumors.8 As reported in the present case, AV tumors are often multiple and/or bilateral.4 Furthermore, regions of atypical tubular hyperplasia characterized by more than a single cell layer forming a solid tubule or papillary projections can also be seen associated with it.1,5 The first report of spontaneous RTTs with AV features in rats occurred nearly 60 years ago,2 and it represented an example of a Mendelian inherited predisposition (autosomal dominant pattern) in which all heterozygous animals develop tumors).3,4 It is now known that its spontaneous occurrence in the Long-Evans (Eker) rat is due to mutations in the tuberous sclerosis gene (Tsc2).10 A tumor exhibiting similar morphologic features has also been observed in SD (Nihon) rats, and it is attributable to mutations in the Birt-Hogg-Dube´ (Bhd) tumor suppressor gene.11 Germline mutations in either of these 2 tumor suppressor genes predispose the rats to multicentric, bilateral renal tubular neoplasia and hyperplasia.6 In the Eker rat, tumors have a later onset and appear vacuolated and chromophilic; conversely, neoplasms in rats harboring the Bhd mutation exhibit an early onset and may have a clear cell, cystic, papillary, chromophobe, or eosinophilic appearance.11
A previous report identified a low incidence of spontaneous AV tumors in one group of F344 littermates.16 AV tumors have been reported sporadically in several strains of young rats used in subchronic toxicity (90-days) studies,6,7,13 as well as in two-year carcinogenicity bioassays.8 Interestingly, they have been observed in control and treated groups,8 and have previously been demonstrated to be spontaneous, nontreatment-related.1,8,9 Additionally, their distinct histological profile clearly helped differentiating them from the conventional chemically induced RTTs.1 Furthermore, in support of the familial nature of these neoplasms, it was not uncommon for multiple tumors to appear within the same toxicologic study where the animals are often littermates,1 in the absence of underlying toxic/proliferative changes.6 Metastases of AV tumors were not reported in the previous studies, at least in the lungs,8 and were not found in the present case. Complete blood count (CBC), serum chemistry and urinalysis were not performed prior to euthanasia. Minimal tubular ectasia accompanied by hyaline casts and multifocal tubular basophilia were noted histologically, and no other changes were found in the organs examined. These findings are compatible with an early stage of chronic progressive nephropathy (or CPN), which is one of the most common spontaneous lesions in rats and mice and it is commonly observed in nearly all male rats.5 Although no association with chronic progressive nephropathy was previously reported,8 the animal in the present case was older compared other animals diagnosed with AV tumors.1,6,11
Given its aforementioned features, it has been proposed to record this distinctive renal tumor phenotype separately from conventional RTTs and allow for appropriate interpretation of the carcinogenic potential of a test article on the kidneys.1,8
Contributing Institution:
Laboratory of Comparative Pathology; Hospital for Special Surgery, Memorial Sloan Kettering Cancer Center, The Rockefeller University, Weill Cornell Medicine.
https://www.mskcc.org/research-areas/programs-centers/comparative-medicine-pathology
JPC diagnosis:
Kidney: Renal cell carcinoma, amphophilic-vacuolar type.
JPC comment:
The contributor provides a concise summary of this infrequently encountered renal tumor. A number of rats have been used as animal models of autosomal dominant gene associated renal tumors, including the Eker and Nihon rats. Others, such as Sprague-Dawley, Fischer 344, and Wistar rats also experience spontaneous proliferative renal lesions. As of this writing, there have been no reports of metastasis, or chemical induction of these tumors.15
In rats, basophilic carcinomas with well organized lobules are the most common carcinomas. While clear cell and papillary carcinomas occur in the rat, they are much less frequent, and anaplastic and sarcomatoid variants are rare. For purposes of classification, both amphophilic vacuolar type neoplasms and oncocytomas should be considered distinct and separate from basophilic tumor types.5
A recent report of three cases of AV tumors in Sprague-Dawley rats participating in a 2-year carcinogenicity study highlight the scientific gaps in our knowledge of this disease pathogenesis and progression. The animals were all in placebo control groups, and so contribute to the evidence for familial disease in these rats.12
A number of histologically similar neoplasms have recently been reported in Tg.Rash2 mice in 26-week carcinogenicity studies. The lesions were adenomas and a carcinoma with amphophilic staining of polygonal cells, and tumors demonstrated cystic and/or papillary patterns. Because these lesions are thought to be familial in rats, a trace back to the parents of affected mice was performed. However, there was no correlation in lesions between generations. The lesions in affected mice all presented with multiple neoplasms, either multiple foci in one kidney, or lesions in both kidneys. These likely arose as spontaneous lesions.14
References:
- Crabbs TA, Frame SR, Laast VA, Patrick DJ, Thomas J, Zimmerman B, Hardisty JF. Occurrence of spontaneous amphophilic-vacuolar renal tubule tumors in Sprague-Dawley rats from subchronic toxicity studies Toxicol Pathol 2013; 41: 866-871.
- Eker R. Familial renal adenomas in Wistar rats?A preliminary report. Acta Pathol Microbiol Scand 1954; 34: 554?562.
- Eker R and Mossige J. A dominant gene for the renal adenomas in the rat. Nature 1961; 189: 858-859.
- Everitt JI, Goldsworthy TL, Wolf DC, Walker CL. Hereditary renal cell carcinoma in the Eker rat: A rodent familial cancer syndrome. J Urol 1992; 148: 1932?1936.
- Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol 2012; 40: 14S-86S.
- Hall WC, Elder B, Walker CL, Cai S, Peters DG, Goodman DG, Ulland BM, Borzelleca JF. Spontaneous renal tubular hyperplastic and neoplastic lesions in three Sprague Dawley rats from a 90-day toxicity study. Toxicol Pathol 2007; 35: 233?241.
- Hard GC, Long PH, Crissman JW, Everitt JI, Yano BL, Bertram TA. Atypical tubule hyperplasia and renal tubule tumors in conventional rats on 90- day toxicity studies. Toxicol Pathol 1994; 22:489?496.
- Hard GC, Seely JC, Kissling GE, Betz LJ. Spontaneous occurrence of a distinctive renal tubule tumor phenotype in rat carcinogenicity studies conducted by the National Toxicology Program. Toxicol Pathol 2008; 36: 388?396.
- Hardisty JF, Banas DA, Gopinath C, Hall WC, Hard GC, Takahashi M. Spontaneous renal tumors in two rats from a thirteen week rodent feeding study with grain from molecular stacked trait lepidopteran and coleopteran resistant (DP-ØØ4114-3) maize. Food Chem Toxicol 2013; 53: 428-431.
- Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat Gen 1995; 9: 70?74.
- Kouchi M, Okimoto K, Matsumoto I, Tanaka K., Yasuba M, Hino O. Natural history of the Nihon (Bhd gene mutant) rat, a novel model for human Birt-Hogg-Dube´ syndrome. Virchows Arch 2006; 448: 463?471.
- Kudo K, Hoshiya T, Nakazawa T, et al. Spontaneous renal tumors suspected of being familial in Sprague-Dawley rats. J Toxicol Pathol. 2012;25:277-280.
- Lanzoni A, Pialia A, Everitt J, Faustinelli I, DeFazio R, Cavaliere L, Cristofori P. Early onset of spontaneous renal preneoplastic and neoplastic lesions in young conventional rats in toxicity studies. Toxicol Pathol 2007; 35: 589?593.
- Paranjpe MG, Belich JL, McKeon ME, et al. Renal tumors in 26-week Tg.Rash2 mice carcinogenicity studies. Toxicol Pathol. 2016;44(5):633-635.
- Seely JC, Hard GC, Blankenship B. Kidney. In: Suttie AW, ed. Boorman's Pathology of the Rat. San Diego, CA:Elsevier. 2018:147-149.
- Thurman JD, Hailey JR, Turturro A, Gaylor DW. Spontaneous renal tubular carcinoma in Fischer-344 rat littermates. Vet Pathol 1995; 32: 419?422.