Signalment:
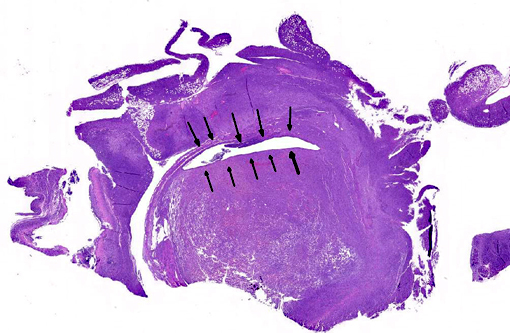
Gross Description:
Histopathologic Description:
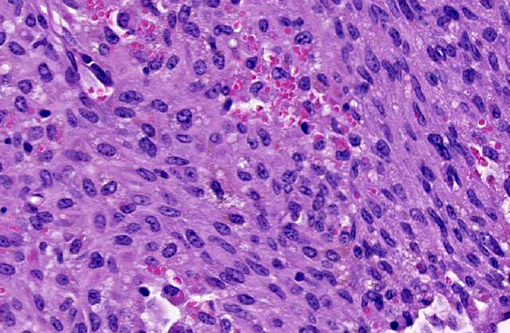
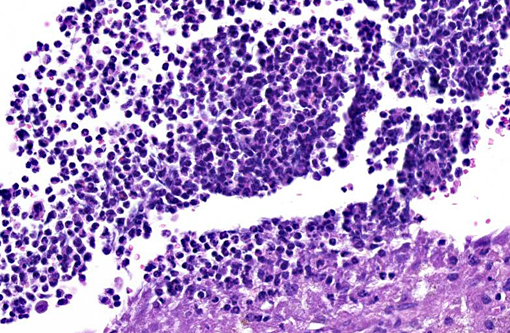
The uterus also has a neoplastic cell population in the endometrium, myometrium and surrounding soft tissue. In some areas the neoplasm has sheets of oval to round cells with a moderate amount of amphophilic cytoplasm and eccentric nuclei. In the uterine wall the cells have a fusiform to spindle morphology. Nuclei are elongated to reniform with marginated chromatin. There are approximately 8 mitotic figures per ten 400X field. Similar neoplastic cells efface the pancreas and are present in the mesometrium, ovary, gall bladder, in abdominal lymph nodes and the spleen. Kidneys have cytoplasmic eosinophilic droplets in the proximal tubular epithelium.
Immunohistochemistry results: Performed on neoplastic aggregates present in the mesovarium and ovary: F4/80: Positive. Smooth muscle actin and desmin: Negative
Gram stain: Uterus: The short bacterial rods are gram negative; occasional gram positive cocci are present.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Differential diagnoses for histiocytic sarcoma include stromal tumors like leiomyoma and Schwannoma, and other hematopoietic cell tumors such as histiocyte-associated lymphomas (HAL) and myelogenous leukemia. Significant populations of histiocytic cells can be present in lymphomas; the macrophages associated with HAL tend to be large and round with abundant cytoplasm. Histiocyte rich lymphomas can be distinguished by clonal rearrangements of Ig heavy chain (B-cell) or T-cell receptor loci in lymphocyte populations versus having IgH and TcR receptor genes in germline (non-rearranged) configuration. This is done by Southern blot analysis. If neoplastic cells lack IgH gene rearrangements, neoplasms of B-cell origin are excluded; however, rarely cells with histiocytic cell morphology have displayed IgH rearrangements. Some histiocyte-rich neoplasms have nodular proliferations of more pleomorphic macrophages increased numbers of mitotic figures and lymphocytes with clonal immune receptor and rearrangements; these may represent tumors with both neoplastic histiocytes and lymphocytes.(5) Immunohistochemistry for T-cell and B-cells were not performed in the submitted case.
Pasteurella is a common pathogen in laboratory mice that has been associated with uterine infections as well as male reproductive, ocular, ear, nasal, skin and mammary gland infections.(8) Peptostreptococcus has not been reported as a cause of spontaneous disease in mice but has been associated with genitourinary tract infections in people.(7)
Mouse Histiocyte markers(9)
| Marker | Function | Cell location | Macrophage expression | Other tissues |
| Arginase 1 | Arginine metabolism | Cytostolic | M2* macrophage | Other tissues |
| CD68 | Lysosome/phagosome A glycoprotein | Cytostolic | Tissue macrophages, thymus, lymph node, Kupffer cells, alveolar macrophages | Also other cells with lysozomes/phagosomes |
| CD163 | Scavenger receptor cysteine rich superfamily | Membrane | Monocytes and tissue macrophages except tingible body macrophages in germinal centers | |
| F4/80 | Immune regulation(8) | Membrane | Tissue macrophage; not splenic white pulp | |
| IAB1 | Actin/calcium binding protein | Cytoplasm and nucleus | All but alveolar macrophage and lymph node dendritic cells | |
| iNO2 | Nitric oxide expression | Cytoplasmic | Activated macrophages via CD4TH1pathway | neutrophils |
| Lysozyme | Innate immune system | Cytoplasmic | Alveolar macrophage, Kupffer cells, Lymph node sinuses | Granulocytes, monocytes |
| MAC 2 | Adhesion molecule | Cytoplasmic and membrane | All | Also various epithelial cells |
| Mac3 | Glycoprotein | Cytoplasm | Macrophages and dendritic cells | Epithelial, megakaryocytes, endothelial cells, granulocytes |
| YM1 | Expression associated with inflammation | Cytoplasm | M2 macrophage, Alveolar macrophages | M2 |
JPC Diagnosis:
1. Uterus, mesometrium, pancreas, mesentery: Histiocytic sarcoma.
2. Uterus: Endometritis, necrotizing, diffuse, severe, with intra- and extracellular bacilli.
Conference Comment:
The contributor provides an excellent overview of histiocytic sarcoma in rodents and its immunohistochemical attributes. Immunohistochemistry has afforded greater clarity in teasing out cellular origin of various histiocytic diseases, effectively eliminating commonly used diagnoses such as malignant fibrous histiocytoma and splenic fibrohistiocytic nodules while identifying the majority of histiocytic proliferations in dogs and cats as Langerhans cell or interstitial dendritic cell origin.(6,11) Only hemophagocytic sarcoma is still recognized as originating from macrophages.(6) Histiocytic proliferative diseases may occur as neoplastic processes or dysregulated inflammatory processes in dogs, while only neoplastic processes are identified in cats.(6)
Histiocytic sarcoma is one of the few neoplasms known to cross the joint space and is often confused with synovial cell sarcoma when present at an articular surface. Synovial cell sarcomas are derived from type B synovial cells, which are specialized fibroblasts that readily attract large numbers of histiocytes. The two are differentiated by the dominant cell population and its CD18 expressivity.(6) Histiocytic sarcoma is one of three typically disseminated neoplasms in rodents, to also include hemangiosarcoma and lymphoma,that are believed to arise synchronously in multiple organs. It is interesting there is no mention of liver involvement in this case, where it occurs so commonly and was often cited as a primary location in older literature.(2)
Pyometra most commonly occurs following estrus in most species, while the uterus is under progesterone influence.(10) In mice, pyometra may also occur secondarily to mucometra, and Klebsiella spp. and Pasteurella spp. are the most commonly cultured organisms.(2,8)
References:
1. Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: Background strains and experimental design. Vet Path. 2012; 49 (1): 85-105
2. Davis BJ, Dixon D, Herbert RA. Ovary, oviduct, uterus, cervix, and vagina. In: Maronpot RR, ed. Pathology of the Mouse. Vienna, IL: Cache River Press, Inc.; 1999:438.
3. Decker JH, Dochterman LW, Niquette AL et al. Association of renal tubular hyaline droplets with lymphoma in CD-1 Mice. Toxicol Pathol. 2012; 40 (4): 651-655
4. Frith CH, Ward JM, Harlmen JH et al. Hematopoietic System. In International Classification of Rodent Tumors: The Mouse. Berlin, Germany: Springer-Verlag; 2001: 430-431
5. Hao X, Fredrickson TN, Chattopadhyay SK. The histopathologic and molecular basis for the diagnosis of histiocytic sarcoma and histiocyteassociated lymphoma of mice. Vet Pathol. 2010; 47 (3): 434-445
6. Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol. 2014;51(1):167-184.
7. Murdoch DA. Gram-Positive Anaerobic Cocci. Clinical Microbiology Reviews 1998; 11 (1): 81-120
8. Percy DH and Barthold SW. Mouse In: Pathology of Laboratory Rodents and Rabbits, 3rd ed. Oxford, England: Blackwell Publishing Ltd; 2007: 3-111
9. Rehg JE, Bush D and Ward JM. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol Pathol. 2012; 40 (2): 345-374
10. Valli VE, Jacobs RM, Parodi AL, Vernau W, Moore PF. Histological Classification of Hematopoietic Tumors of Domestic Animals. 2nd series. Vol VIII. Washington, D.C.: Armed Forces Institute of Pathology American Registry of Pathology; 2002:48-49.
11. van den Berg TK, Kraal G. A function for the macrophage F4/80 molecule intolerance induction. Trends in Immunology 2005; 26 (10): 506-509