Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 24
May 2nd, 2018
CASE II: IP17-300 (JPC 4101145)
Signalment: Juvenile, undetermined sex, Nile tilapia (Oreochromis niloticus), pisces.
History: In a group of 50,000 fish, after 21 days of culture, with an approximate weight between 0.8 and 1 gram, a considerable amount of mortality was reported. Some of these fish had difficulty swimming and were found on the pondâs surface. There were whitish spots distributed throughout the body. At least 300 fish were found dead on the surface and countless more were found on the pondâs bottom. Wet mounts of gills and skin (including fins), were performed to calculate the mean intensity for monogeneans, trichodinids and Ichthyophthirius multifiliis; the values obtained were 5, 15 and 47 parasites per infected fish respectively. The prevalence for these same parasites was 46%, 53.3% and 100% respectively.
Gross Pathology: Multifocal to coalescing raised pinpoint 2mm white spots covered most of the skin, gills and oral cavity; in some fish these pinpoint lesions affected the cornea as well.
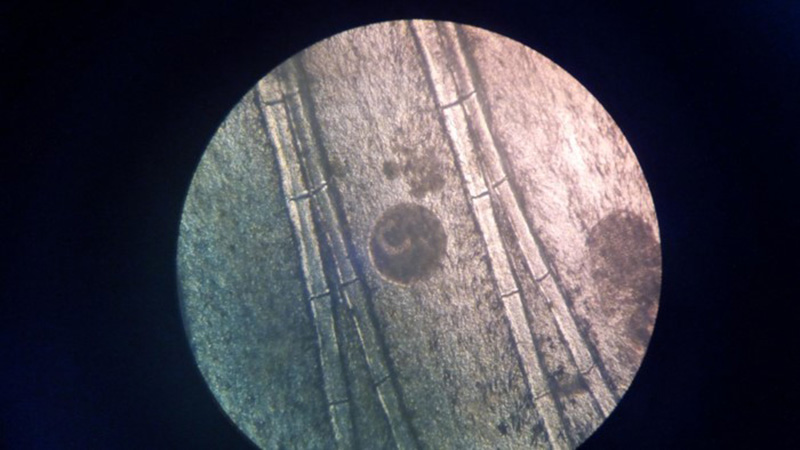
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): Wet mounts of gill clippings obtained at necropsy demonstrated large numbers of round, 50-300 μm diameter theronts and trophonts consistent with Ichthyophthirius multifiliis.
Microscopic Description:
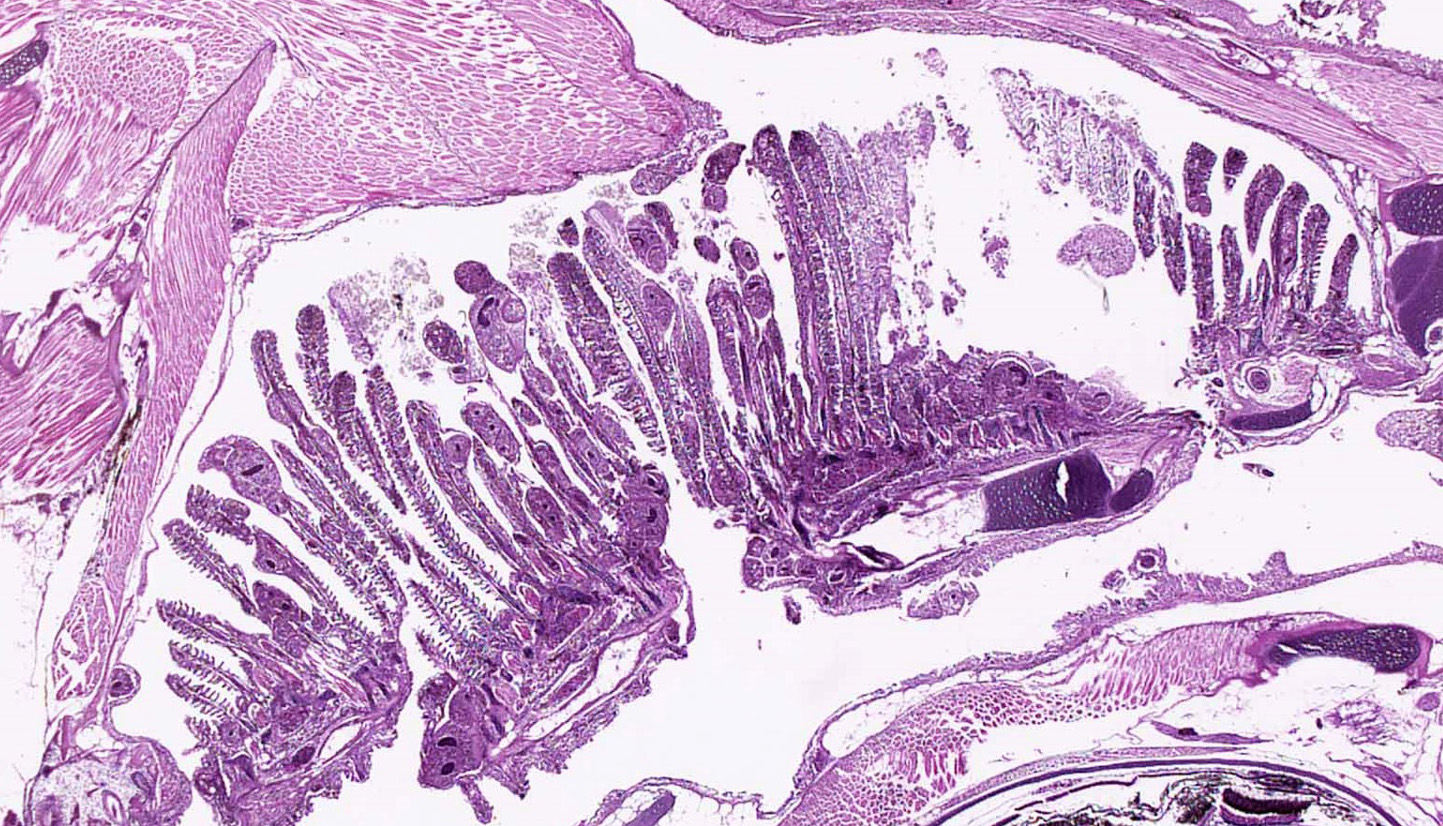
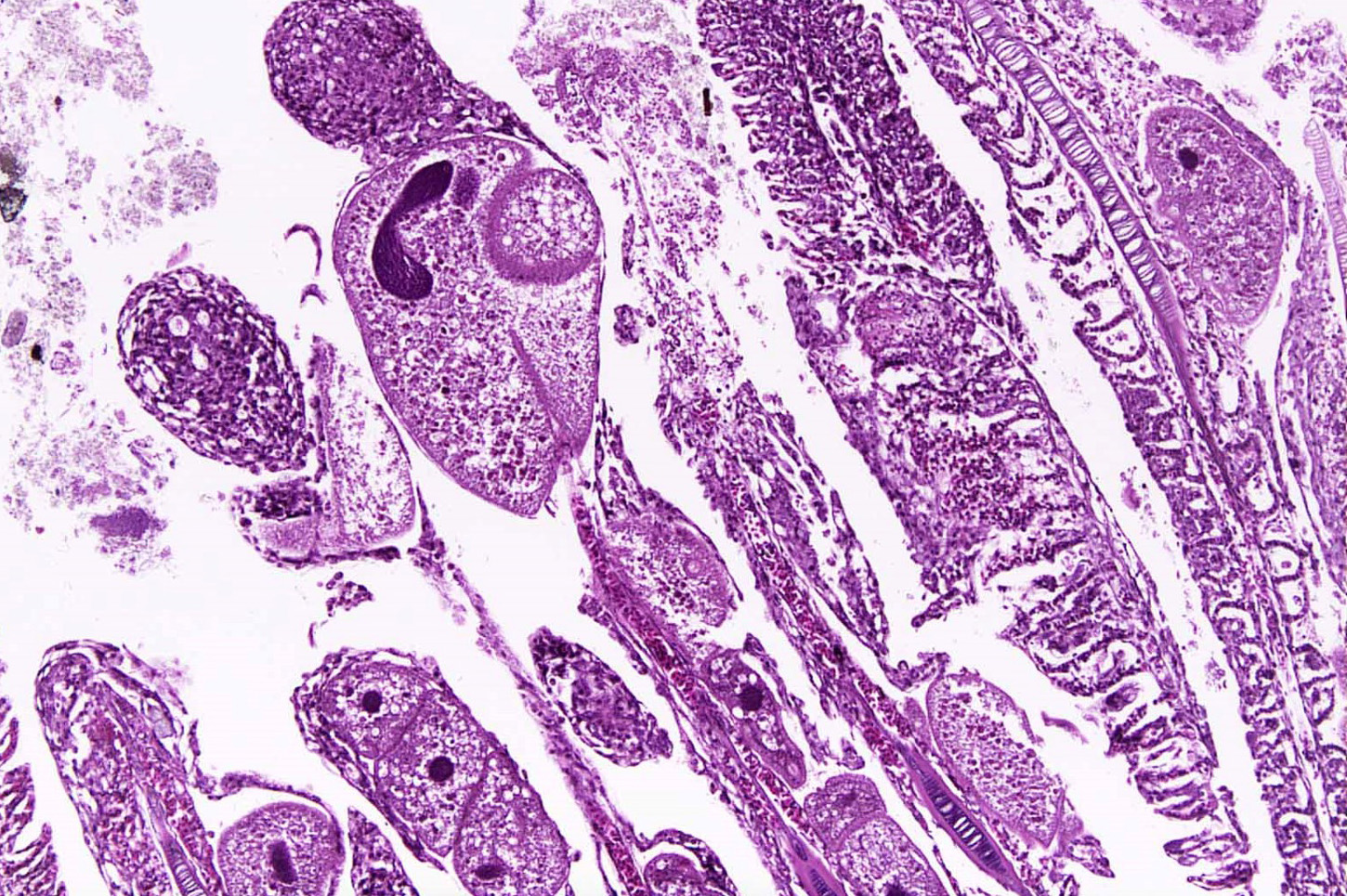
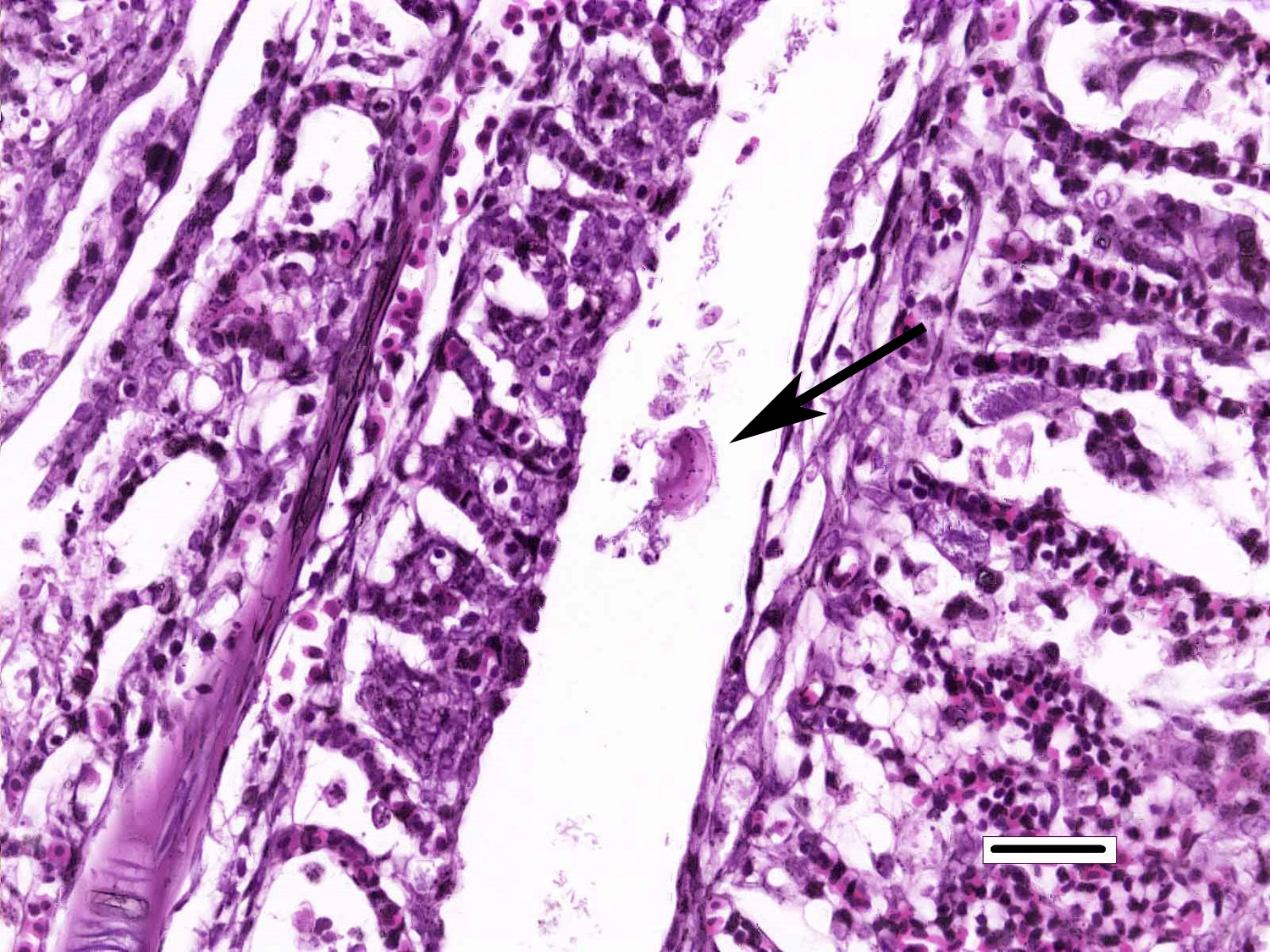
Gills: Multifocally, there is moderate hyperplasia of the gill epithelium with blunting and fusion of secondary lamellae and numerous irregularly round, single?cell, up to 200 um diameter, intraepithelial protozoal cysts with a 1?2 um thick hyaline wall, abundant, finely granular to vacuolated basophilic cytoplasm containing numerous host erythrocytes, and a 30 x100 um, crescent-shaped, deeply basophilic macronucleus (trophont). Goblet cell hyperplasia was also observed. Variable amounts of mucus and necrotic debris admixed with filamentous bacteria was present in some sections. Attached to the lamella or freely between them, numerous saucer shaped, hemispheric, dumbbell shaped, or sac like or flattened cylindrical protozoa, consistent with trichonids, were also observed.
Skin and oral cavity: Multifocally, there are nodular foci within the epidermis that are composed of hyperplastic epithelium that piles up to 6 to 9 cell layers. Hyperplastic epithelium often surrounds previously described intraepithelial protozoa.
Contributorâs Morphologic Diagnosis:
Gill: Epithelial hyperplasia, nodular, moderate, diffuse, with numerous protozoa (trophonts and theronts) consistent with Ichthyophthirius multifiliis and trichodinids.
Skin and oral cavity: Epithelial hyperplasia, nodular, multifocal, moderate with protozoa (trophonts and theronts) consistent with Ichthyophthirius multifiliis.
Contributorâs Comment: Histologic findings were similar in all fish examined. In addition to proliferative branchitis and dermatitis, affected fish exhibited a similar lesion in the oral cavity associated with organisms identical to those observed in the gills. Ichthyophthirius multifiliis is a ciliated protozoan parasite that infects the skin and gills of freshwater fish and causes âIchâ or white spot disease. The life cycle begins with a small migratory and infective stage known as theront attaching to the epidermis and gills, where it feeds and continue its development to a trophont, which elicits a reactive response (epidermal hyperplasia). At this stage, trophonts increase dramatically in size due to enlargement of their macronucleus, production of food vacuoles and liposomes, and formation of new mucocysts. The trophont breaks through the epithelium, drops off the host, and forms a capsule (tomont) that adheres to the bottom of the tank. Tomonts undergo binary fission within the cyst to produce tomites which break through the cyst and eventually become infective motile theronts. Ichthyophthirius multifilis are 75 μm to 1 mm in diameter and uniformly ciliated with a crescent-shaped nucleus. I. multifiliis causes localized lymphocytic infiltration, focal necrosis, and varying degrees of epithelial proliferation in the skin and gills. In severe cases, sloughing of the epidermis has been observed.1,2,3,10 In experimentally infected channel catfish (Ictalurus punctatus), infection of the peritoneal cavity has been reported; three possible enterance routes were speculated: penetration through esophageal wall, penetration of the pneumatic duct (a structure connecting the esophagus and swim bladder), or retrograde migration from the anus.5 In saltwater fish Cryptocaryon irritans has the same life cycle as I. multifilis, where it is referred as âmarine ichâ and produces similar lesions.
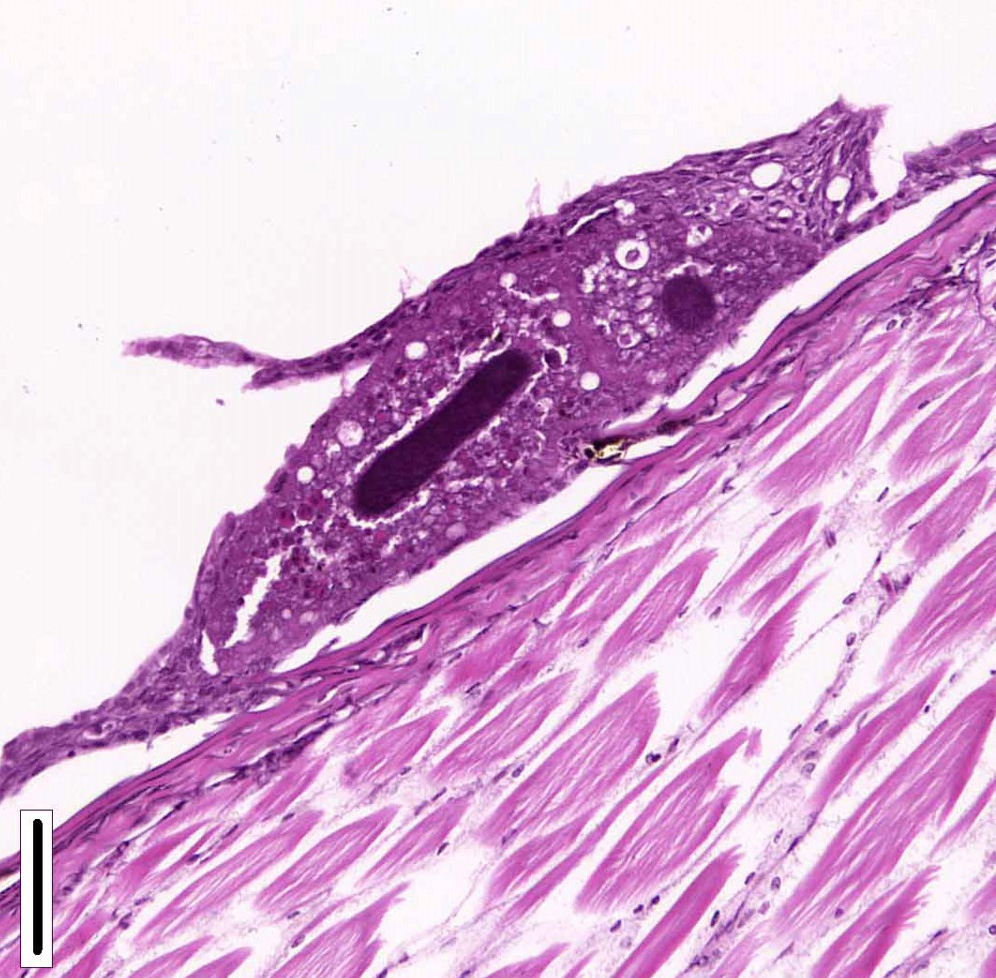
Conversely, trichodinas/trichodinids are mobile peritrich ciliates that attach temporarily to the substrate while feeding and have been found in both freshwater and marine fish. They are found in the skin and gills. Although they are typically considered commensal organisms, they may become numerous in stressed or debilitated fish. Depending on the orientation of the parasites in tissue sections, they may appear as saucer shaped, hemispheric, dumbbell shaped, and sac like or flattened cylindrical organisms. Heavy infection with this parasite, has been associated with excessive secretion of mucous, and can result in hypertrophy and hyperplasia of gill epithelium with subsequent fusion of secondary gill lamellae.1,2
In addition to I. multifilis and trichodinids, several other potential etiologic agents were observed microscopically that could have contributed to some extent to the lesions in gills, skin and oral cavity. Depending on the slide, there were also few monongeneans, filamentous bacteria and epitheliocystis. In this group of fish, I. multifilis is believed to be the primary pathogen when one takes into account the previously stated prevalence and mean intensity.
Fish parasites are an integral part of water ecosystems and they are common in wild and cultured populations of fish. It is well known that fish live in balance with parasites, however, this balance can be broken by stressors such as sudden changes in water quality (i.e. temperature, oxygen, etc) and poor husbandry (i.e. high stocking densities, excessive handling, etc.). Therefore, diseases caused by parasites are much more frequently manifested in cultured fish, which suffer from numerous stress factors that influence their ability to effectively protect themselves against parasitic infections. Infections caused by protozoan and metazoan parasites occur very frequently in cultured fish and can cause significant economic losses to fish farms due to mortality, but parasites may also exert considerable impact on growth and behavior of fish, on their resistance to other stress factors, susceptibility to predation, etc. Many of these parasites can cause severe injury to different organs and tissues and they can provide portals of entry for bacteria in fish, generating co-infections.4,7,9
JPC Diagnosis: 1. Gills, pharynx, skin: Epithelial hyperplasia, blunting and fusion of secondary lamellae and numerous embedded protozoal ciliates (theronts) consistent with Ichthyophthirius multifiliis and free trichodinids, Nile tilapia (Oreochromis niloticus), pisces.
- Gills: Epitheliocystis, rare.
- Gills: Filamentous bacilli, multifocal.
- Gills: Monogenean, single.
Conference Comment: The gills of teleosts are the most vulnerable structures they possess; their external location ensures close association with the external environment and any contaminates or parasites that inhabit it. External protozoan and monogenean trematode parasites have a particular affinity for the gills because they have a rich blood supply and thus provide a nutrient-rich environment. Additionally, the gills are often the route of entry for various bacterial and viral agents (lymphocystis or Herpesvirus salmonis for example) which spread from the branchial vessels hematogenously and systemically either via leukocyte trafficking or cell-free dissemination. The gills are composed of two main parts: the primary lamellae (which extend out from the branchial arch) and the secondary lamellae (which protrude as smaller projections out from the primary lamellae). Within the primary lamellae are: epithelium, endothelium, pillar cells and supporting stroma (made up of fibrous and cartilaginous connective tissues). Admixed are specialized cells such as: mucous cells, salt cells, eosinophilic granule cells and fixed macrophages. When injured, the inflammatory response is limited, and the earliest microscopic lesions are swelling and degeneration of the lamellar epithelial cells, or edema of the subepithelial connective tissue. In general, lamellar edema and epithelial necrosis are the result of acute exposure to direct acting toxins or chemical pollutants such as heavy metals, red tides, phytoplankton or jellyfish and ultimately lead to hemorrhage. On the other hand, lamellar hyperplasia often results from chronic exposure to lower levels of a toxicant and can assume several morphologic forms: clubbing of secondary lamellae, mucous cell hyperplasia or metaplasia, and ultimately lamellar fusion. With good water quality and a modicum of time, most gill lesions heal.6,8
Table 1: Gross and microscopic differentials for Ichthyophthirius multifilis6,8
|
Protozoan |
||
|
Cryptocaryon irritans (marine ich) |
Saltwater fish |
Penetrates the epithelium; saltwater equivalent of Ichthyophthirius multifilis |
|
Trichodina spp. |
Marine or freshwater fish |
Grossly similar to Ichthyophthirius multifilis; identified on wet mount as disk shaped organism scooting on the surface of tissues |
|
Chilodonella spp. |
|
Same life cycle and gross pathology as Ichthyophthirius multifilis; more severe tissue damage |
|
Amyloodinium sp. (marine velvet disease) |
Warm water marine fish (elasmobranches (sharks, rays) and teleost (ray fin fish) |
Dinoflagellate; affects gills, skin, and eyes; larger than Ichthyophthirius multifilis |
|
Piscinoodinium spp. (freshwater velvet disease, rust disease) |
Freshwater fish |
Freshwater analogue of Amyloodiniosis |
|
Ichthyobodo spp. (Ischthyobodo necator complex) |
Immunosuppressed and young fish; freshwater primarily |
Smallest ectoparasite of fish (size of red blood cell); epithelial hyperplasia with increased mucus production (makes fish bluer and extra slimey) |
|
Fungal/algae |
||
|
Saprolegniales sp. (water mold) |
Freshwater (especially estuarine tropical fish) |
Cottony, proliferative growth on skin or gills |
|
Bacterial |
||
|
Epitheliocystis sp. |
Freshwater and marine fish |
Intracellular, Gram-negative; causes epithelial and dermal cell enlargement |
|
Viral |
||
|
Lymphocystis (pscine iridovirus) |
|
Hypertrophied fibroblasts with basophilic intracytoplasmic inclusion bodies |
Icthyophthirius multifilis (also known as âichâ or white spot disease) is the largest protozoan parasite in fish; trophozoites can reach 100 µm in diameter and have a prominent oval or horseshoe-shaped nucleus. Ich is common in aquarium and hatchery-reared freshwater fish and can result in respiratory impairment in severely infected fish. Microscopically, the trophozoites are found in the skin or gill lamellae surrounded by epithelial hyperplasia. I. multifilis has a direct life cycle in which encysted trophozoites (trophonts) leave the fish and settle at the bottom of the tank where, in their tomont form, they divide into numerous motile tomites (theronts). It is the motile theront form that infects the skin of the fish. Their total life cycle only takes 4 days but can be quicker in warmer water temperatures.
Trichodina spp. is a saucer-shaped, 50 µm in diameter, peritrichal ciliated protozoan with a macro- and a micronucleus. Microscopically, it appears as a characteristic ring of interlocking denticles. Low numbers are not typically associated with disease and are frequently environmental contaminates. However, in increased numbers, with concurrent disease, or in an immunosuppressed host, they can cause increased skin and gill mucus and respiratory distress. Trichodina spp. have a simple life cycle and reproduce by binary fission.6,8
Contributing Institution:
Departamento de PatologÃa (Pathology Department)
Facultad de Medicina Veterinaria y Zootecnia
Universidad Nacional Autónoma de México
Mexico city, Mexico
http://fmvz.unam.mx/fmvz/departamentos/patologia/acerca.html
References:
- Bruno DW, Nowak B, Elliot DG. Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections. Aqua. Org. 2006; 70:1-36.
- Dar SA, Kaur H, Chishti MZ, Ahmad F, Tak IR, Dar GH. First record of protozoan parasites in cyprinid fish, Schizothorax niger Heckel, 1838 from Dal Lake in Kashmir Himalayas with study on their pathogenesis. Microb Pathogenesis. 2016; 93:100-104.
- Gardiner CH, Fayer R, Dubey JP. An Atlas of Protozoan Parasites in Animal Tissues. 2nd Washington DC: Armed Force Institute of Pathology, American Registry of Pathology, 1998: 16-17.
- Kotob MH, Menanteau-Ledouble S, Kumar G, Abdelzaher M, El-Matbouli M. The impact of co-infections on fish: a review. Vet Res. 2016; 47(98):1-12.
- Maki JL, Brown CC, Dickerson HW. Occurrence of Ichthyophthirius multifiliis within the peritoneal cavities of infected channel catfish Ictalurus punctatu. Dis Aquat Org. 2001; 44:41â45.
- Noga EJ. Fish Disease Diagnosis and Treatment. 2nd Ames, IA: Wiley-Blackwell; 2010:129-148.
- Pantoja MFW, Neves RL, Dias RDM, Marinho GBR, Montagner D, Tavares-Dias M. Protozoan and metazoan parasites of Nile tilapia Oreochromis niloticus cultured in Brazil. MVZ Córdoba. 2012; 17(1):2812-2819.
- Roberts RJ. Fish Pathology. 4 ed. West Sussex, UK: Wiley-Blackwell; 2012: 76-85, 159, 309.
- Scholz T. Parasites in cultured and feral fish. Vet Parasitol. 1999; 84:317-335.
- Wei JZ, Li H, Yu H. Ichthyophthiriasis: emphases on the epizootiology. Lett Appl Microbiol. 2013; 57:91â101.