Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 14
9 January, 2019
CASE IV: Case 1 (JPC 4103659).
Signalment: 2-year old, male, NCTR Sprague Dawley (CD) rat, (Rattus rattus)
History: This male rat was part of a 2-year carcinogenesis study that included prenatal and perinatal exposure to the test article. The animal survived to terminal sacrifice and was euthanized via CO2 inhalation.
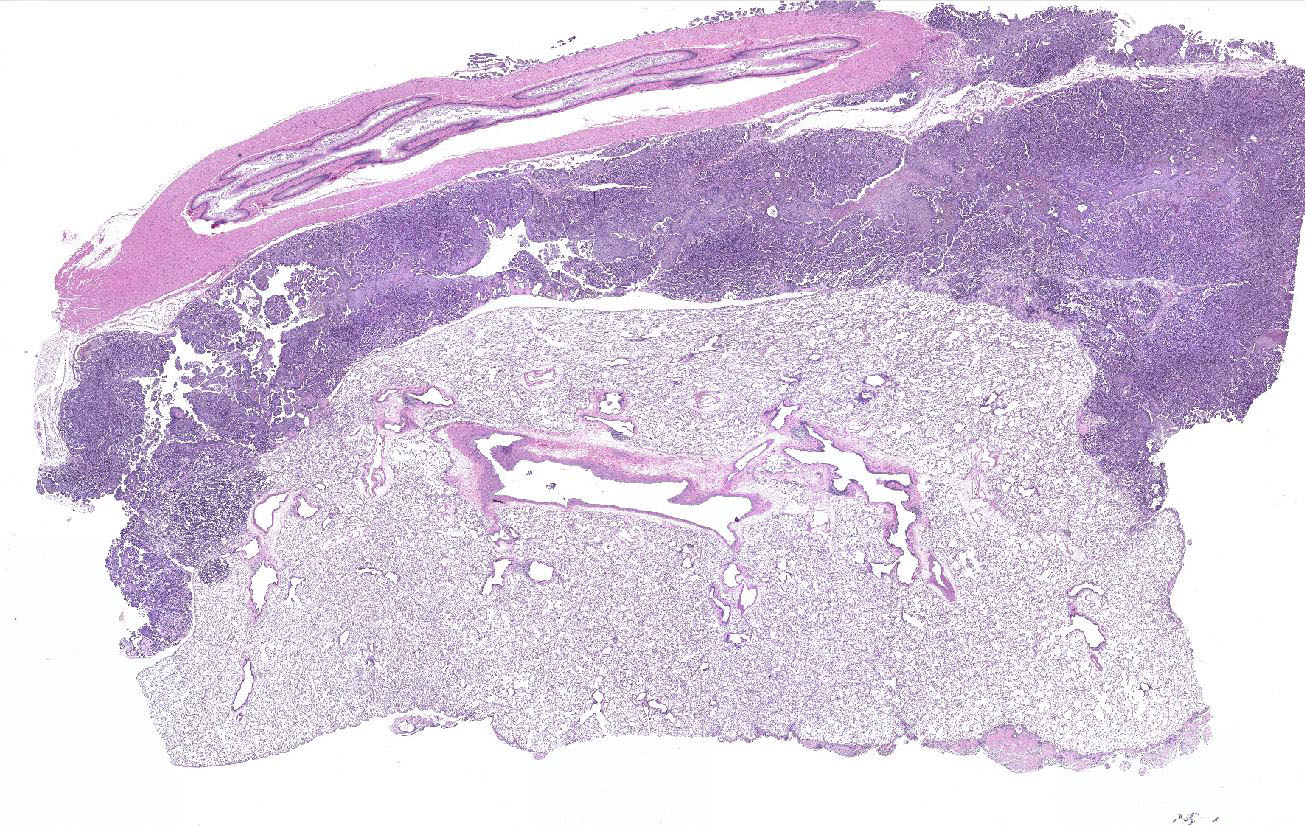
Gross Pathology: At necropsy, an extensive tumor was identified within the thoracic cavity that involved the lung, pleura, pericardium, diaphragm, and rib.
Laboratory results: None.
Microscopic Description:
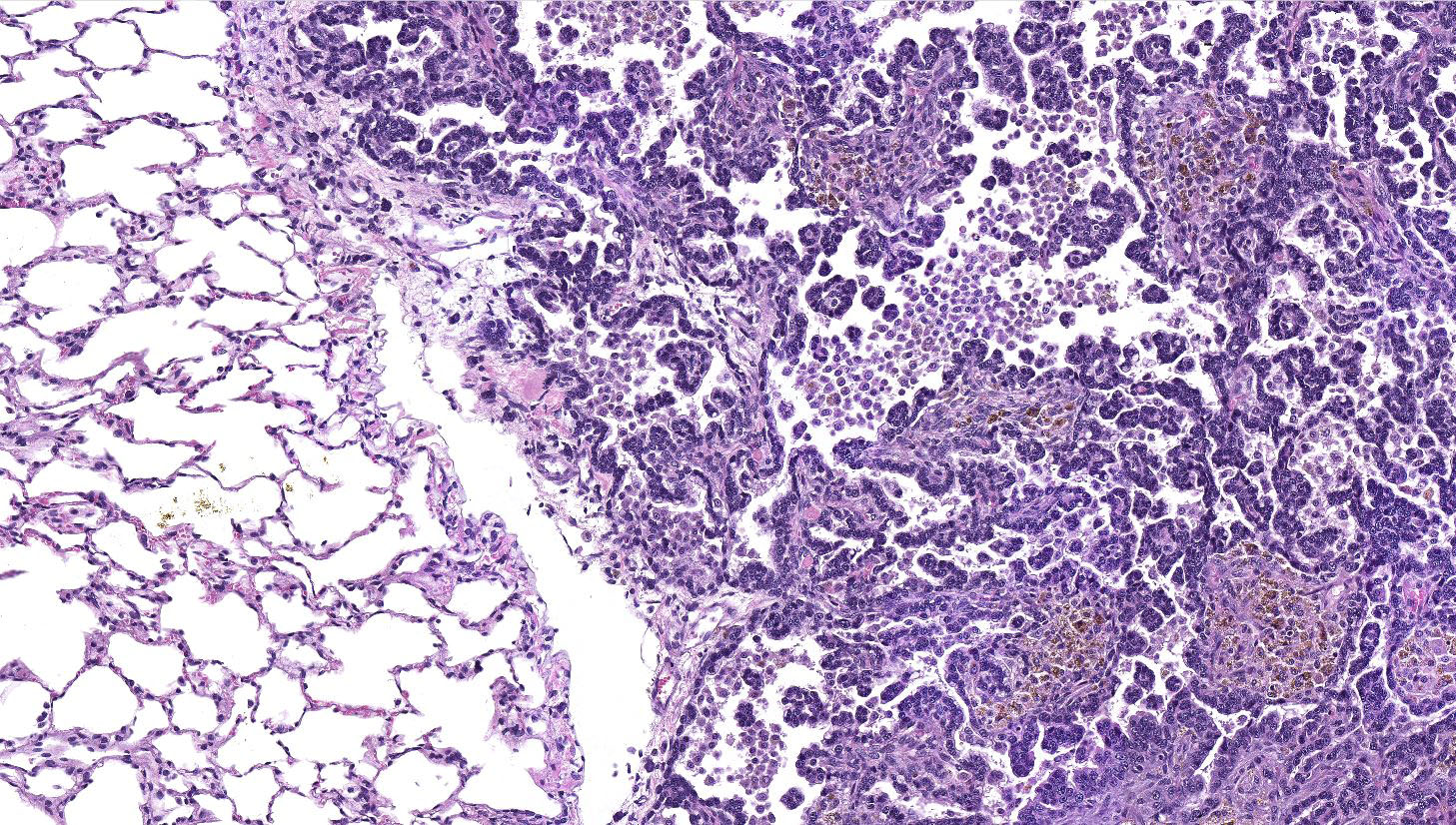
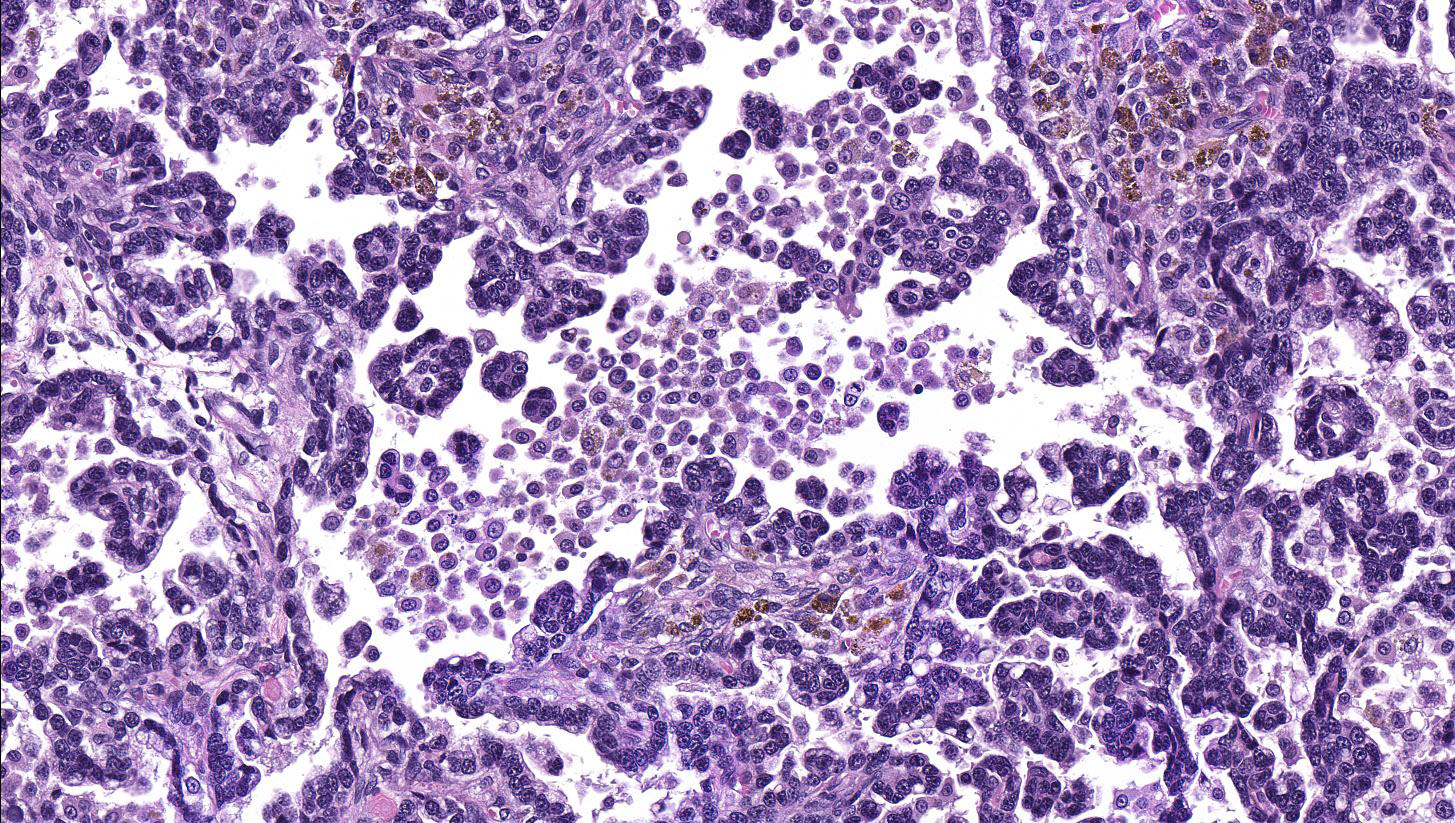
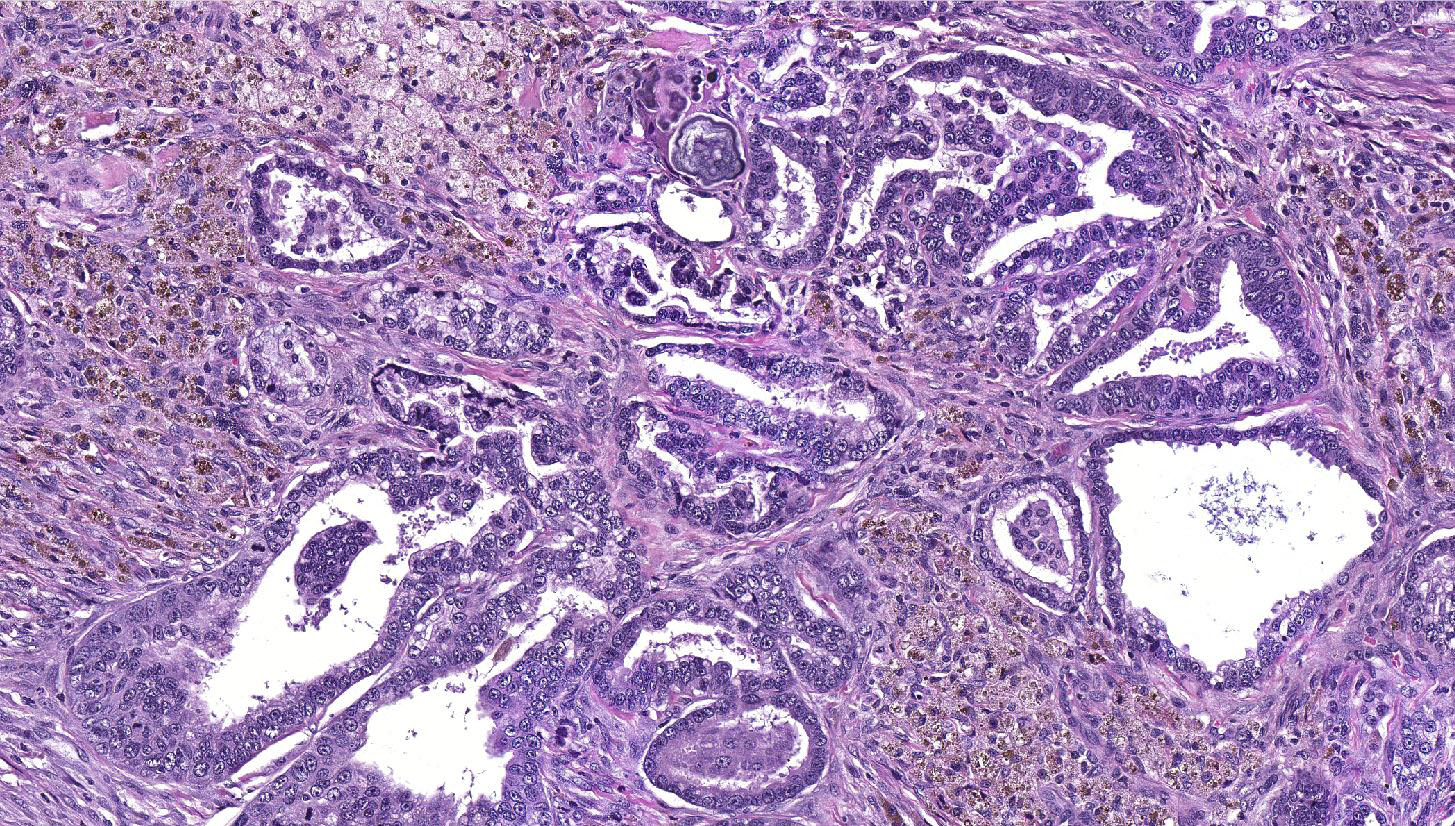
Lung: a multifocal, unencapsulated, expansile, neoplasm is diffusely expanding the pleural surface, compressing the adjacent lung parenchyma and adhering to the costal pleura of the adjacent rib. The neoplasm is composed of epithelial cells, arranged in tubules and cords, as well as papillary and alveolar-like structures that are supported by small amounts of fine, fibrovascular stroma. Neoplastic cells are cuboidal to columnar, with moderate amounts of finely fibrillar, eosinophilic cytoplasm and a single, round to oval, central to basilar nucleus, with 1-3 variably distinct nucleoli and stippled chromatin. Anisocytosis and anisokaryosis are minimal and the mitotic rate is low with 0-2 mitotic figures per 40X field. Some areas within the neoplasm contain abundant fibrous connective tissue (schirrous response) infiltrated by large numbers of hemosiderin-laden macrophages. Large influxes of foamy, alveolar macrophages are frequently observed within the neoplasm. Multifocal areas of hemorrhage, emphysema and edema are also present.
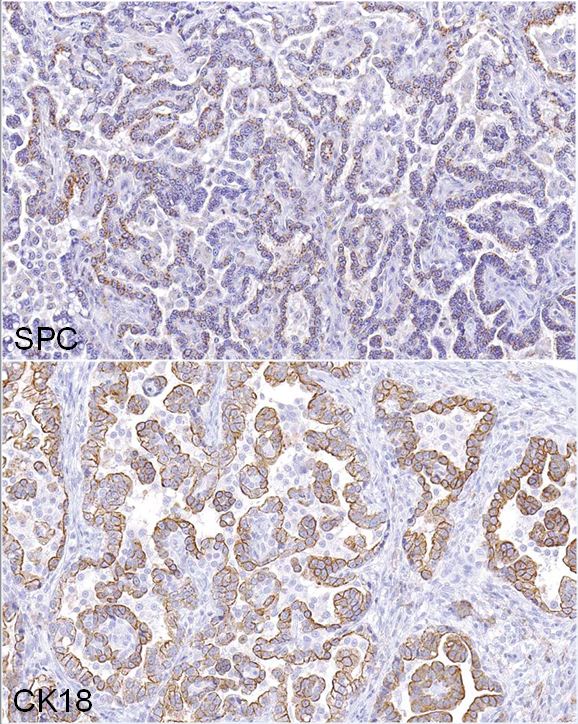
Immunohistochemistry: neoplastic cells exhibit a similar staining pattern to that of alveolar-bronchiolar (AB carcinoma) with positive cytoplasmic staining with SPC and cytokeratin 18 antibodies and negative staining with vimentin and CC10.
Contributor’s Morphologic Diagnosis:
Lung: Alveolar-bronchiolar carcinoma (bronchiolo-alveolar carcinoma)
Contributor’s Comment: Alveolar-bronchiolar carcinomas (AB carcinomas), are the most commonly induced pulmonary malignancies in rodent studies conducted by the National Toxicology Program (NTP).2 However, the current literature now refers to these types of tumors as bronchiolo-alveolar carcinomas.2 Although the cell of origin for bronchiolo-alveolar tumors remains somewhat controversial, the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) guidance document on rodent respiratory lesions indicates that bronchiolo-alveolar carcinoma may originate from club cells (formerly known as Clara cells) or type II alveolar cells, but more commonly arise from type II alveolar cells.2,5 These types of tumors typically invade the lung parenchyma as poorly circumscribed, nodular masses that may occupy an entire lung lobe and infiltrate adjacent tissues.1 However, extra-pulmonary AB carcinomas that are largely mediastinal have been reported.4
Several growth patterns for AB carcinoma have been described in the rat, these include: Alveolar (glandular) patterns - consisting of cuboidal to columnar cells forming glandular structures, papillary growth - cuboidal to columnar cells forming papillary structures supported by a connective tissue core, tubular patterns - prominent elongated tubules, solid- composed of tightly arranged round cells with no visible space in-between or mixed patterns where multiple patterns are apparent. Marked pleomorphism, areas of squamous metaplasia or abundant fibrosis (schirrous response) are additional features that have been described in rats and mice as well as large influxes of macrophages into the tumor and adjacent alveoli, as observed in this case.1,5
This case was presented to a pathology working group (PWG) because the study and reviewing pathologists were torn between a diagnosis of AB carcinoma or malignant mesothelioma. Immunohistochemistry was applied to help determine the cell of origin. The neoplasm exhibited IHC staining characteristics similar to a positive AB Carcinoma control that was pulled from the NCTR archives. The majority of neoplastic cells demonstrated positive cytoplasmic staining for SPC (surfactant protein C, alveolar type II cells) and CK18 (cytokeratin 18, epithelial cells) markers, confirming that the neoplasm was most likely an epithelial tumor arising from type II alveolar cells. The cells lining the pleural surface of the neoplasm appeared to represent a layer of reactive mesothelium, as these cells stained positive with both CK18 and vimentin stains, similar to a malignant mesothelioma control also pulled from the NCTR archives; most of the neoplasm was negative for vimentin. Based on the IHC profile, the tumor was diagnosed an AB carcinoma. However, it should be noted, that not every pathologist that reviewed these findings were convinced of the diagnosis.
Contributing Institution:
EPL, Inc., P.O. Box 12766, Research Triangle Park, NC 27709 http://epl-inc.com/
JPC Diagnosis: Lung: Alveolar-bronchiolar carcinoma, papillary type.
JPC Comment: A review of the archives of the National Toxicology Program (NTP) almost a decade ago identified the lung as the organ that is third most common target site of chemical carcinogens in the female rat (with liver and mammary gland preceding it) and as an uncommon site in male rats.2 Inhalation and gavage studies account for 70% of tumors at approximately 35% each, with water-dosing, IP, topical, and in utero exposure accounting for the remainder. Spontaneous A/B neoplasms of the lung occur at incidences of less than 4% in both genders of F344 rats, but over 90% of chemically induced pulmonary neoplasm. The most common site of pulmonary metastases in these rats, interestingly, the skin.2
Alveolar-bronchiolar adenocarcinoma is considered one of the best models for non-small cell lung cancer (NSCLC) in humans. K-RAS, EGFR, and TP53 are the three most commonly altered genes “driver mutations” in NSCLC, with TP53 genes exhibit the highest frequency of mutations in lung tumors (both squamous cell and NSCLC) in smokers.
Differentials for alveolar-bronchiolar adenocarcinoma in the rat should include AB adenomas, acinar carcinomas, adenosquamous carcinoma, mesothelioma, and neoplasms metastatic to the lung.5 AB adenomas, which may represent a continuum with AB carcinoma, are often diagnose based on size, as well as a lack of cellular pleomorphism or atypia as well as a lack of invasive growth.5 Acinar carcinoma grow in a glandular pattern utilizing pre-existent alveolar walls, and may contained mixed populations of pleomorphic cuboidal cells, mucous cells, or ciliated cells. Adenosquamous carcinoma has areas of malignant squamous cells or is composed entirely of squamous cell carcinoma. Metastatic foci of primary adenocarcinoma from other organs may be multifocal and often present within vessels or in perivascular locations. Finally, mesothelioma may be the most difficult of these tumors to differentiate from A/B carcinoma due to its similar predilection to grow along pleural surfaces or within the mediastinum, as well as the variability of cellular morphology.5
References:
- Boorman GA, Eustis SL. In: Boorman GA, ed. Pathology of the Fisher Rat. San Diego, CA: Academic Press, Inc., 1990: 351-357.
- Dixon D, Herbert RA, Kissling GE, Brix AE, Miller RA, Maronpot R. Summary of chemically induced pulmonary lesions in the National Toxicology Program (NTP) toxicology and carcinogenesis studies. Toxicolog Pathol, 36: 428-439, 2008.
- Hong, H, Hoenerhoff MJ, Ton T, Herbert R, Kissling GE, Hooth MJ, Behl M, Witt KL, Smith-Roe SL, Sills RC, Pandiri AR. Kras, Egfr, and Tp53 mutations in B6C3F1/N Mouse and F344/NTac rat alveolar/bronchiolar carcinomas resulting from chronic inhalation exposure to cobalt metal. Toxicol Pathol 2015; 43(6):872-882.
- Howroyd P, Allison N, Foley JF, Hardisty J. Apparent Alveolar Bronchiolar Tumors Arising in the Mediastinum of F344 Rats. Toxicologic Pathology, 37: 351-358, 2009.
- Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinhausen S, Rosenbruch M, Tellier P, Wohrmann T. Proliferative and Nonproliferative Lesions of the Rat and Mouse Respiratory Tract. Toxicologic Pathology, 5S-73S, 2009.