Signalment:
18-year-old, thoroughbred mare (
Equus caballus).The mare had a several year history of a polyp in the nasal cavity. The mass was
surgically removed and submitted as an excisional biopsy.
Gross Description:
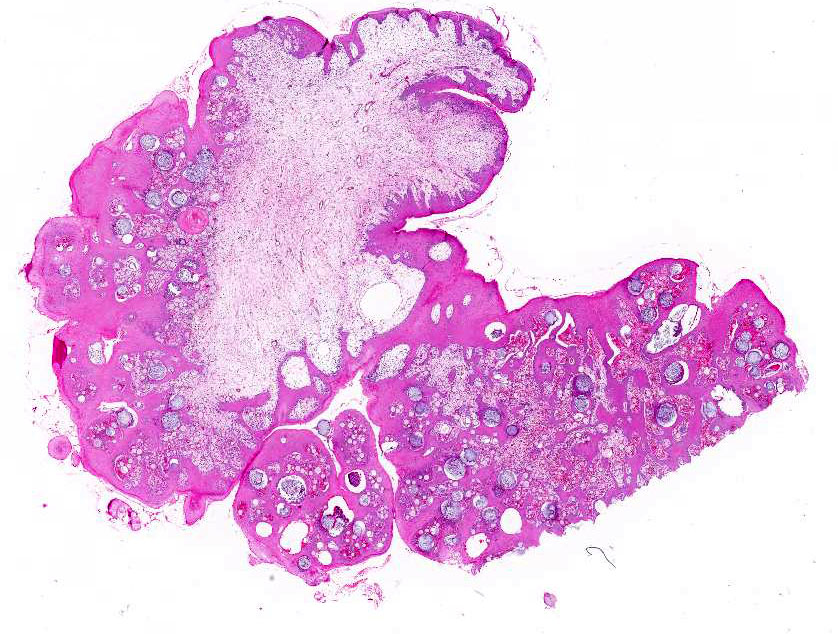
The biopsy sample comprised a 2 x 1.5 x 1 cm smooth, pink-tan, spongy mass with multifocal
ulcers and a narrow stalk at one edge.
Histopathologic Description:
Representative
sections of the tissue comprise a polypoid proliferation of loose fibrovascular
tissue covered by a hyperplastic, acanthotic stratified squamous epithelium
with multifocal erosions and an eosinophilic exudate of mucin admixed with
necrotic neutrophils, debris and erythrocytes. Numerous protistan organisms at
varying stages of development are embedded within the surface exudate,
epithelium, and submucosa, which is infiltrated by moderate numbers of
lymphocytes, plasma cells, macrophages, fewer neutrophils, and variable amounts
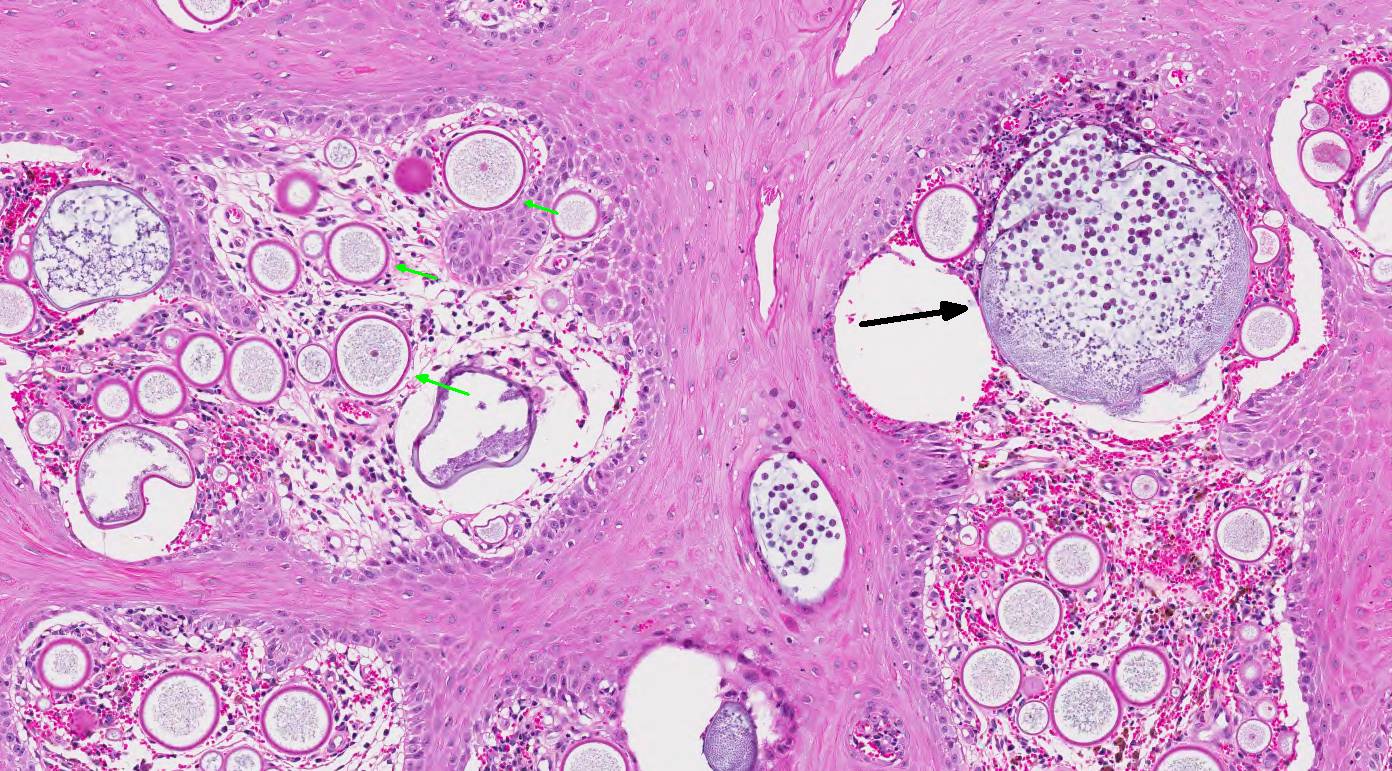
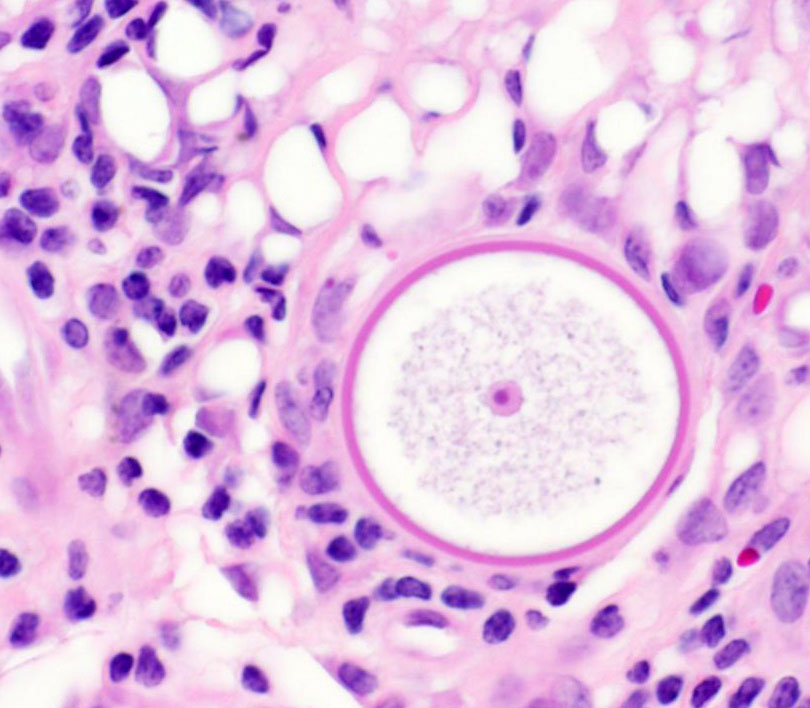
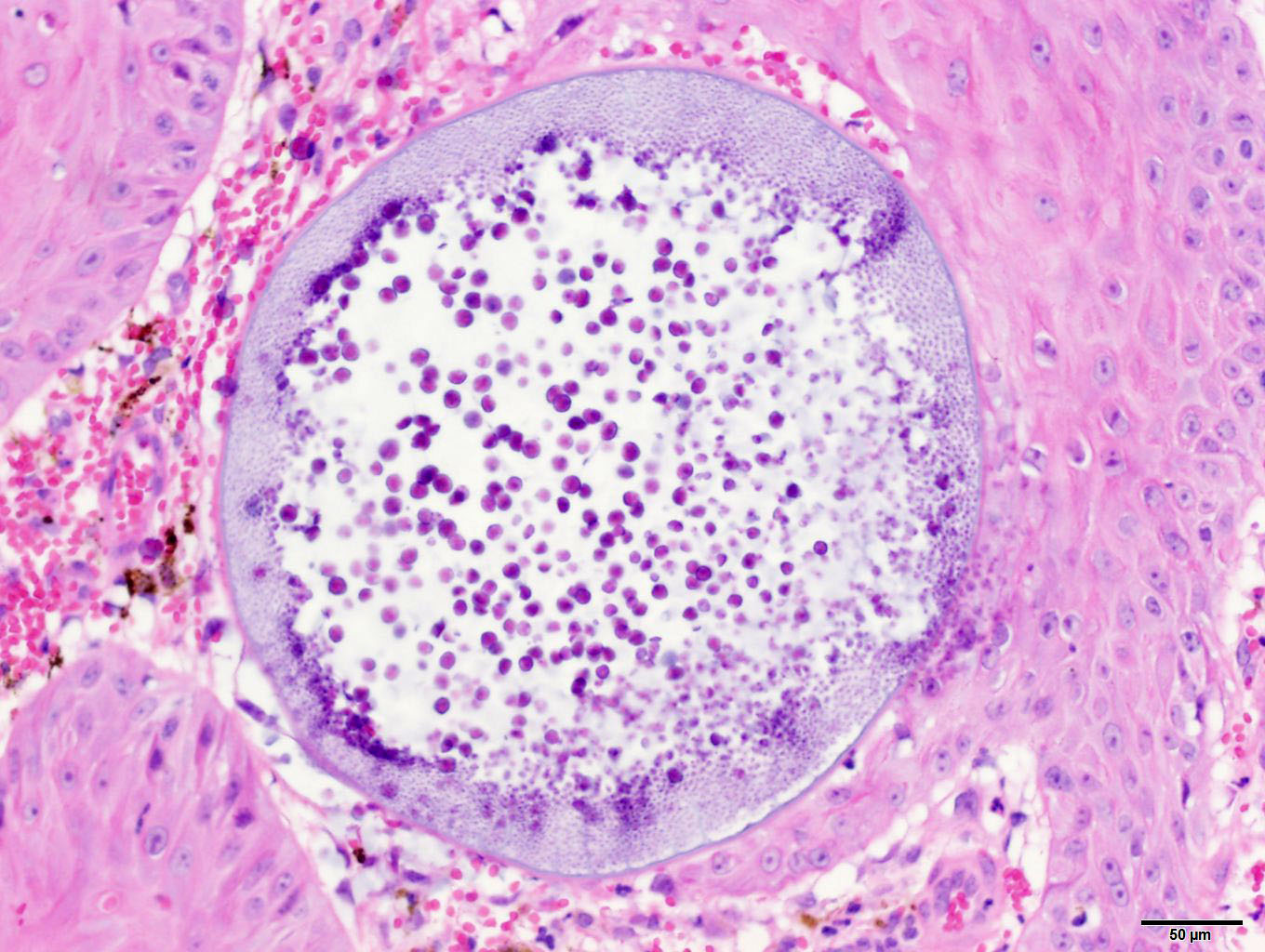
of hemorrhage and edema. Immature sporangia (trophocytes) are round and 10-100
μm diameter, with a 2 μm thick eosinophilic wall, lacy amphophilic
cytoplasm, and a single, central, round karyosome (nucleus). Intermediate
sporangia lack a nucleus and contain innumerable immature sporangiospores
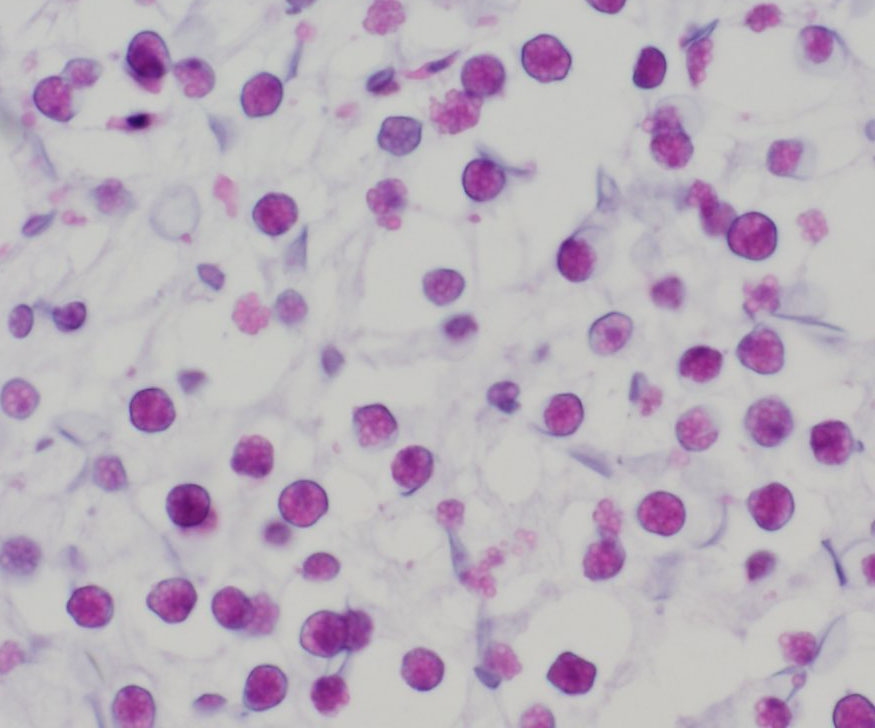
(endospores). Immature endospores are 1-4 μm diameter, with a thin wall,
scant to moderate amounts of lightly basophilic cytoplasm, and irregularly
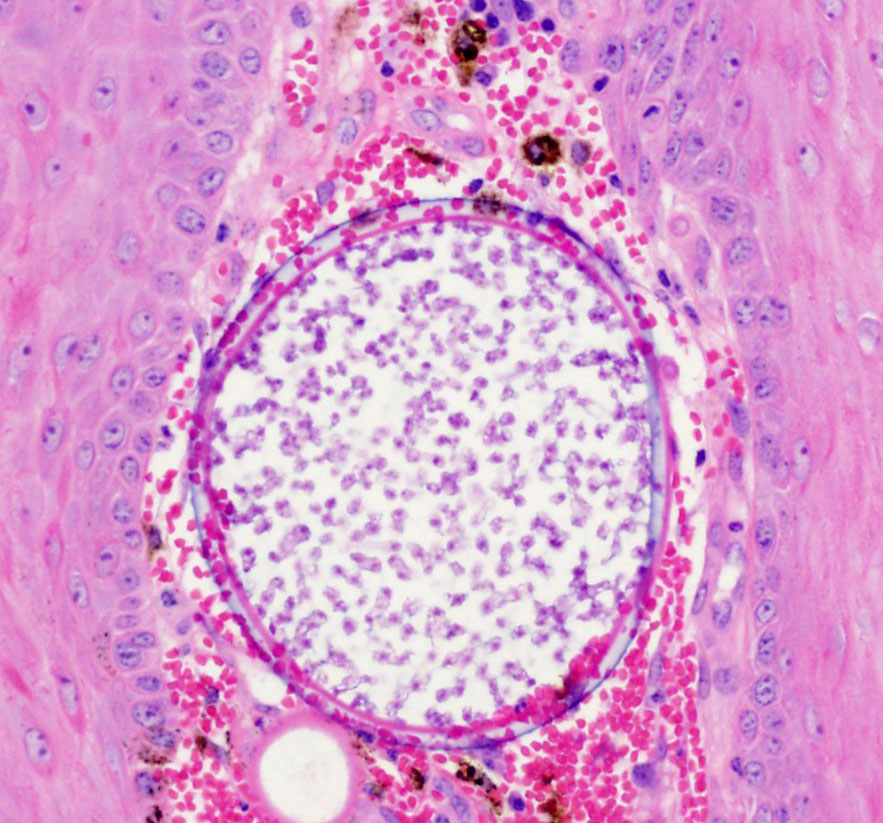
round, para-central eosinophilic structures. Mature sporangia located
throughout the epithelium are spherical and 100-400 μm diameter, with a
2-5 μm thick, bilamellar, outer eosinophilic and inner amphophilic wall.
The mature sporangia contain mature endospores or a mixture of mature and
immature endospores. Mature endospores are 5-8 μm diameter spherical
structures with a thin unilamellar wall, scant clear cytoplasm, and multiple,
central, round eosinophilic bodies. Within mature sporangia, immature
endospores are often located peripherally or emerge unilaterally from the wall
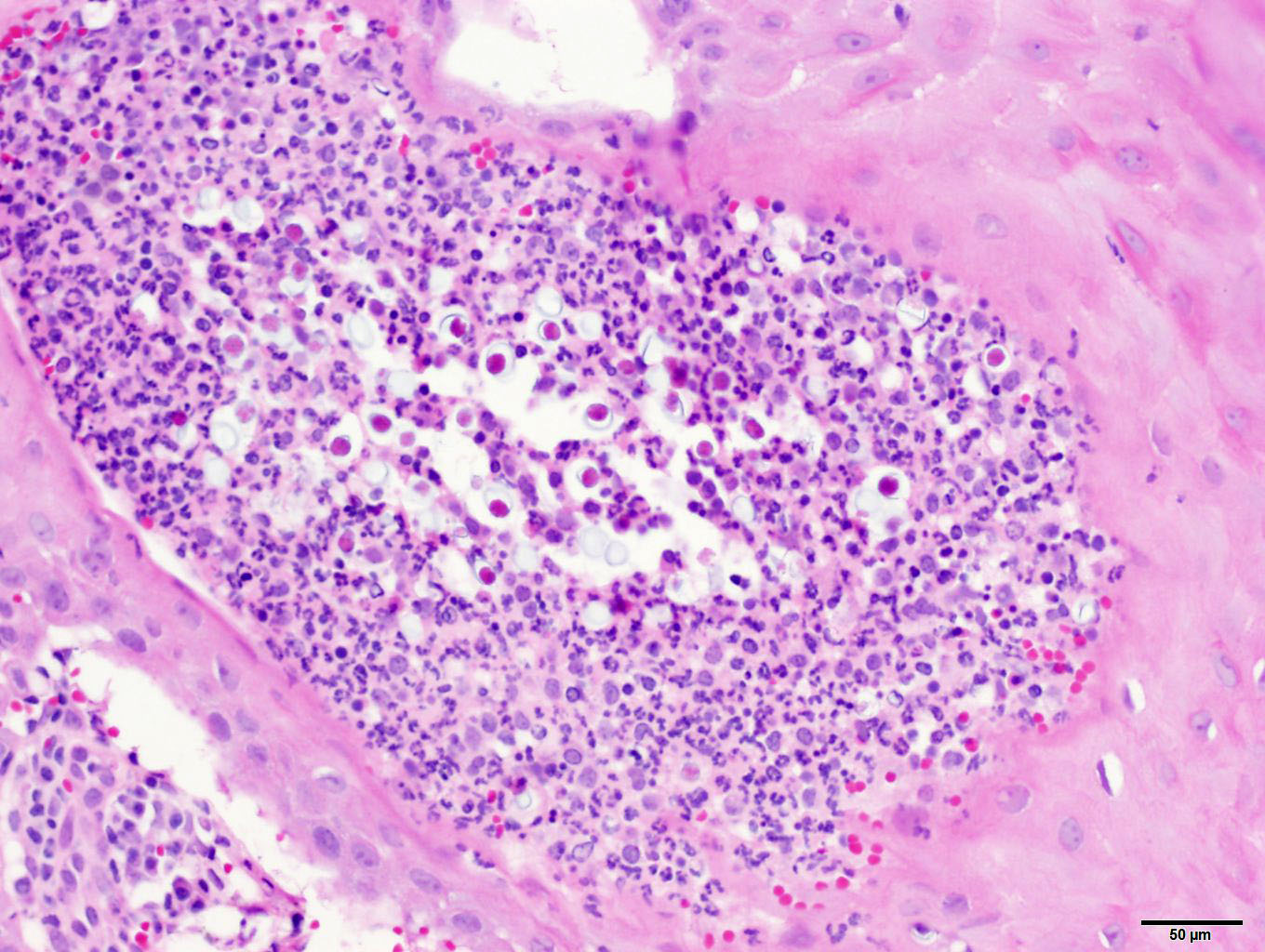
of the sporangium. Mature sporangia are multifocally ruptured, releasing mature
endospores through an apical pore in the epithelium or into the adjacent
stroma, and surrounded by dense aggregates of neutrophils with fewer
lymphocytes, plasma cells, and macrophages.
Morphologic Diagnosis:
Horse, nasal mass: Chronic polypoid and suppurative
rhinosinusitis with protistan sporangia and endospores (consistent with
Rhinosporidium
seeberi).
Lab Results:
N/A
Condition:
Nasal rhinosporidiosis/Rhinosporidium seeberi
Contributor Comment:
Rhinsporiduim
seeberi is an uncommon cause of rhinitis and nasal polyps in humans, dogs,
cats, horses, cattle and waterfowl.
3, 4, 7, 9, 10, 11, 12 The
organism was originally identified in tissue extracted from nasal polyps of
people in Argentina, and has since most commonly been diagnosed in tropical
climates such as India and Sri Lanka.
3 It has been reported
sporadically in horses from the Americas and Europe.
2, 9, 10, 11,
Patients with nasal rhinosporidiosis may
be asymptomatic, or present with clinical signs that include respiratory noise,
sneezing, nasal discharge, and epistaxis.
2, 3, 4 If masses become
large, obstruction of the nasal passage can occur.
1, 3 Examination
of the nasal cavity reveals a unilateral polypoid mass protruding from the
nasal mucosa, though laryngeal masses in association with
R. seeberi
have been reported in horses.
2, 11 Close inspection of the surface
of the mass may reveal small, white foci. In humans, there are reports of
nasal, ocular, laryngeal and genital rhinosporidiosis.
3
Histologic
examination reveals a fibro-vascular polyp covered by stratified squamous to
pseudostratified ciliated columnar epithelium. Sporangia in varying stages of
development are observed throughout the fibrovascular and epithelial components
of the polyp. Identification of sporangia with endospores in a polypoid nasal
mass is diagnostic for
R. seeberi.
7 Organisms are readily
observed in H&E-stained sections; however, histochemical stains, such as
GMS and PAS, can be used to highlight the walls of the sporangia.
5
Polymerase chain reaction (PCR) and DNA sequence analysis of tissue samples can
be used to confirm the etiologic agent.
2
The classification of
R. seeberi
has long been controversial. The organism was once considered to be protozoan,
fungus, or a cyanobacterium. More recently, phylo-genetic analysis categorized
R.
seeberi as a eukaryote within the class
Mesomycetozoa, a group of
aquatic protistan parasites.
5, 6
The pathogenesis
of rhinosporidiosis is poorly understood, though stagnant water appears to be
the reservoir for
R. seeberi. The proposed mechanism of infection
involves pre-existing defects in the epithelial barrier of the nasal passage,
allowing mature endospores to enter the nasal mucosa from contaminated water.
3
Once within host tissues, endospores localize to the sub-epithelial connective tissues and mature
into immature sporangia (trophocytes). Immature sporangia have thick,
unilamellar eosinophilic walls, a central nucleus, and fine cytoplasm. As
immature sporangia become intermediate sporangia, a bilamellar wall develops,
the nucleus is lost, and the cytoplasm condenses to form immature
sporangiospores (endospores). Intermediate sporangia eventually become larger,
contain both immature and mature endospores, and are localized within the
superficial epithelium. At this stage, the sporangia are considered mature and
will continue to house developing endospores until release. Mature endospores
contain multiple, central eosinophilic bodies, and are released from sporangia
through apical pores in the epithelium into the nasal passage. Infective,
mature endospores released into the nasal passage are then excreted into the
environment along with nasal secretions.
Surgical
removal of any visible polyps is the treatment of choice for nasal rhino-sporidiosis.
Excision is typically curative; however, recurrence is reported.
2, 3, 7
A diagnosis of concurrent nasal adeno-carcinoma and rhinosporidiosis was
reported in a cat housed on a horse farm.
1
In this horse,
infectious organisms were embedded within a polypoid proliferation, grossly and
histologically resembling a nasal polyp. Because rhinosporidiosis in humans is
associated with polypoid proliferations, the authors speculate the
proliferative (polypoid) response in this horse may be secondary to chronic
infection and inflammation, rather than represent a primary nasal polyp. Free
endospores within the submucosa often elicit a pyo-granulomatous to
granulomatous response; however, in this case, the predominant inflammatory
infiltrates were neutrophils admixed with lymphocytes, plasma cells, and
macrophages.
For this horse, travel history, clinical
signs, laboratory findings and post-operative follow-up were not provided by
the submitting veterinarians.
JPC Diagnosis:
Nasal mass:
Rhinitis, polypoid, chronic-active, diffuse, severe, with numerous protistan
sprorangia and endospores, thoroughbred,
Equus caballus
Conference Comment:
The contributor provides an exceptional review of the epidemiology, clinical
presentation, gross lesions, histopathologic findings, and differential
diagnosis for
Rhinosporidium seeberi in horses. Conference participants
were impressed by the tremendous numbers of protistan endospores and striking
variation in the size of the sporangia in the section. Despite slight slide
variability, conference participants noted chronic mixed inflammation with
hemorrhage, edema, and granulation tissue in the subepithelial connective
tissue and stalk of the nasal polyp.
Rhinosporidium
seeberi
is one of the few pathogens which reproduce by endo-sporulation.
3
The endospore is implanted in tissues and grows into much larger and variably
sized sporangia. As sporangia grow and mature, endospores are produced and
released into the surrounding tissue generating marked pyogranulomatous
inflammation and effectively reinitiating the pathogens lifecycle.
3
Conference participants discussed other endosporulators of veterinary
significance including:
Prototheca wickerhamii,
Chlorella sp.,
Coccidioides
sp., and
Batrachochytrium dendrobatidis. Based on the size of the
sporangia and observation of endo-sporulation, the differential diagnosis in
this case includes
Coccidioides sp. (see 2016-2017 WSC 4
Case 4). The endospores of
Rhinosporidum seeberi contain inner granules, while
Coccidioides
sp. endospores do not. Also,
R. seeberi endospores completely stain with
Periodic acid-Schiff (PAS), while only the walls of the endospores stain in
Coccidioides
sp.
8 However, finding large sporangia in a polypoid nasal mass of a
horse is typically considered diagnostic for rhinosporidiosis.
1,3
References:
1. Brenske BM,
Saunders GK. Concurrent nasal adenocarcinoma and rhinosporidiosis in a cat.
J
Vet Diagn Invest. 2010; 22:155-157.
2. Burgess HJ,
Lockerbie BP, Czerwinski S, Scott M. Equine laryngeal rhinosporidiosis in
western Canada.
J Vet Diagn Invest. 2012; 24(4):777-780.
3. Caswell J,
Williams K. Respiratory system, In: Maxie MG, ed.
Jubb, Kennedy, and
Palmers Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA:
Elsevier Saunders; 2016: 579-586.
4. Das S, Kashyap
B, Barua M, Gupta N, Saha R, Vaid L, Banka A. Nasal rhinosporidiosis in humans:
New interpretations and a review of the literature of this enigmatic disease.
Med
Mycol. 2011; 49:311-315.
5. Easley JR,
Meuten DJ, Levy MG, Dykstra MJ, Breitschwerdt EB, Holzinger EA, Cattley RC.
Nasal rhinosporidiosis in the dog.
Vet Pathol. 1986; 23:5056.
6. Fredricks DN,
Jolley JA, Lepp PW, Kosek JC, Relman DA.
Rhinosporidium seeberi: A human
pathogen from a novel group of aquatic protistan parasites.
Emerg Infect Dis.
2000; 6(3):273-282.
7. Herr RA, Ajello
L, Taylor JW, Arseculeratne SN, Mendoza L. Phylogenetic analysis of
Rhinosporidium
seeberis 18S small-subunit ribosomal DNA groups this pathogen among
members of the protoctistan Mesomycetozoa clade
. J Clin Microbiol. 1999;
37:27502754.
8. Jones TC, Hunt
RD, King NW. Diseases caused by fungi. In:
Veterinary Pathology. 6
th
ed. Baltimore, MD: Williams and Wilkins; 1997: 527.
9. Kennedy FA,
Buggage RR, Ajello L. Rhinosporidosis: A description of an unprecedented
outbreak in captive swans (
Cygnus spp.) and a proposal for revision of
the ontogenic nomenclature of
Rhinosporidium seeberi.
J Med Vet Mycol.
1995; 33:157-165.
10. Leeming G,
Hetzel U, Campbell T, Kipar A. Equine rhinosporidiosis: An exotic disease in
the UK.
Vet Rec. 2007; 160:552-554.
11. Moisan PG, Baker
SV. Rhinosporidiosis in a cat
. J Vet Diagn Invest. 2001; 13:352-354.
12. Myers DD, Simon
J, Case MT. Rhinosporidiosis in a horse.
J Am Vet Med Assoc. 1964;
145:345-347.
13. Nollet H,
Vercauteren G, Martens A, Vanschandevijl K, Schauvliege S, Gasthuys F,
Ducatelle R, Deprez P. Laryngeal rhinosporidiosis in a Belgian warmblood horse.
Zoonoses Public Health. 2008; 55:274-278.
Ramachandra
Rao PV, Jain SN, Hanumantha Rao TV. Animal rhinosporidiosis in India with case
reports.
Ann Soc Belge Med Trop. 1975; 55(2);119-124