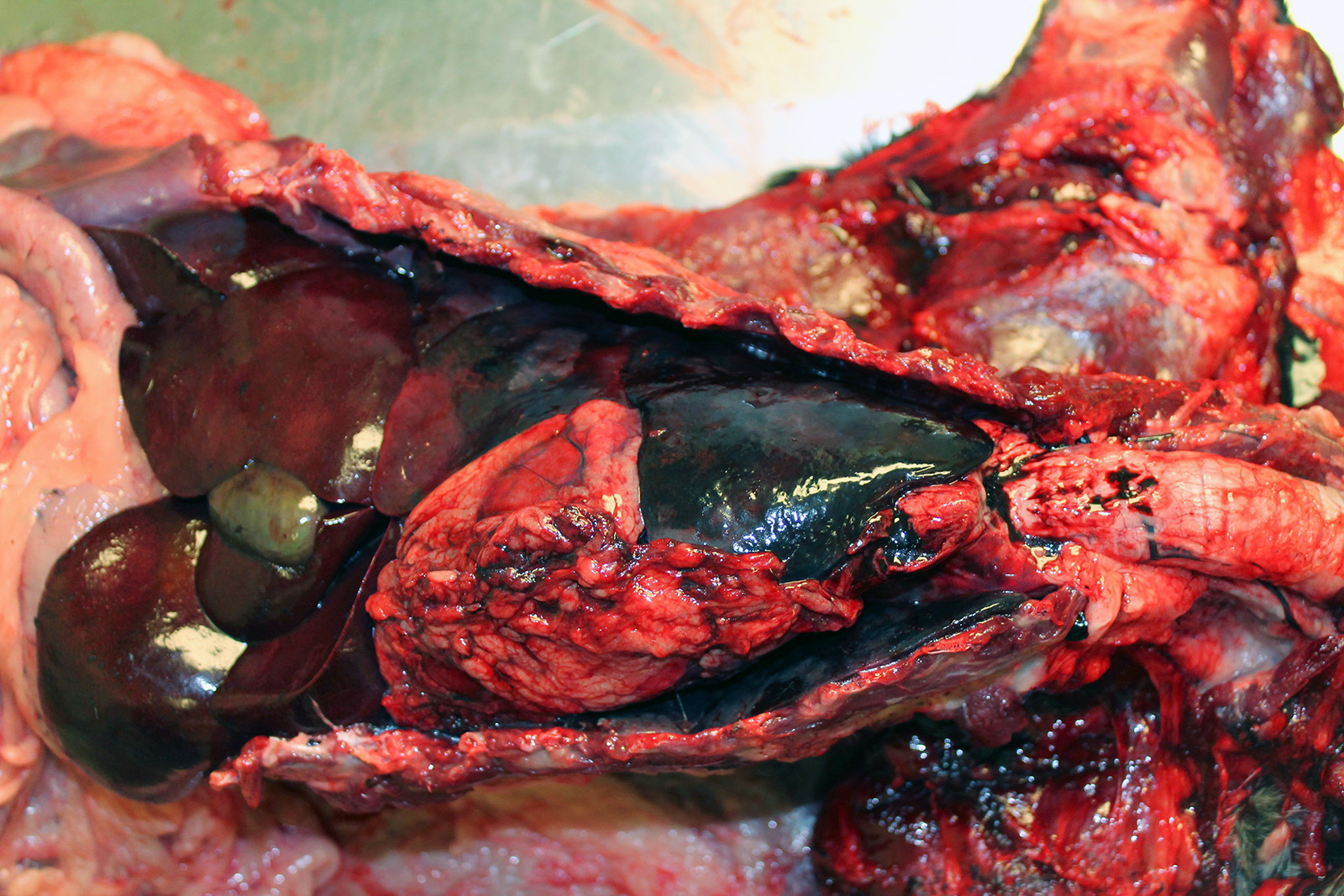
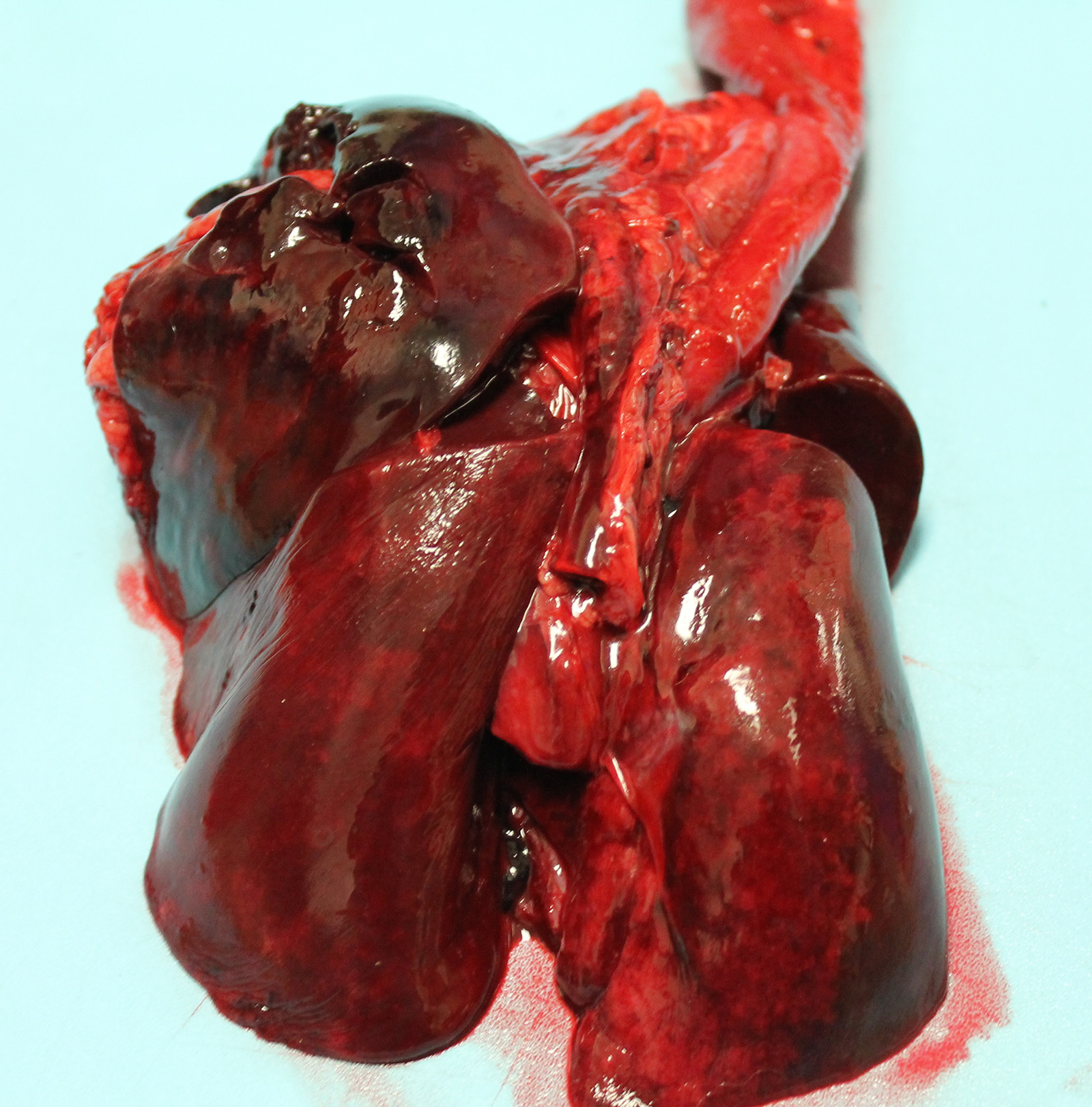
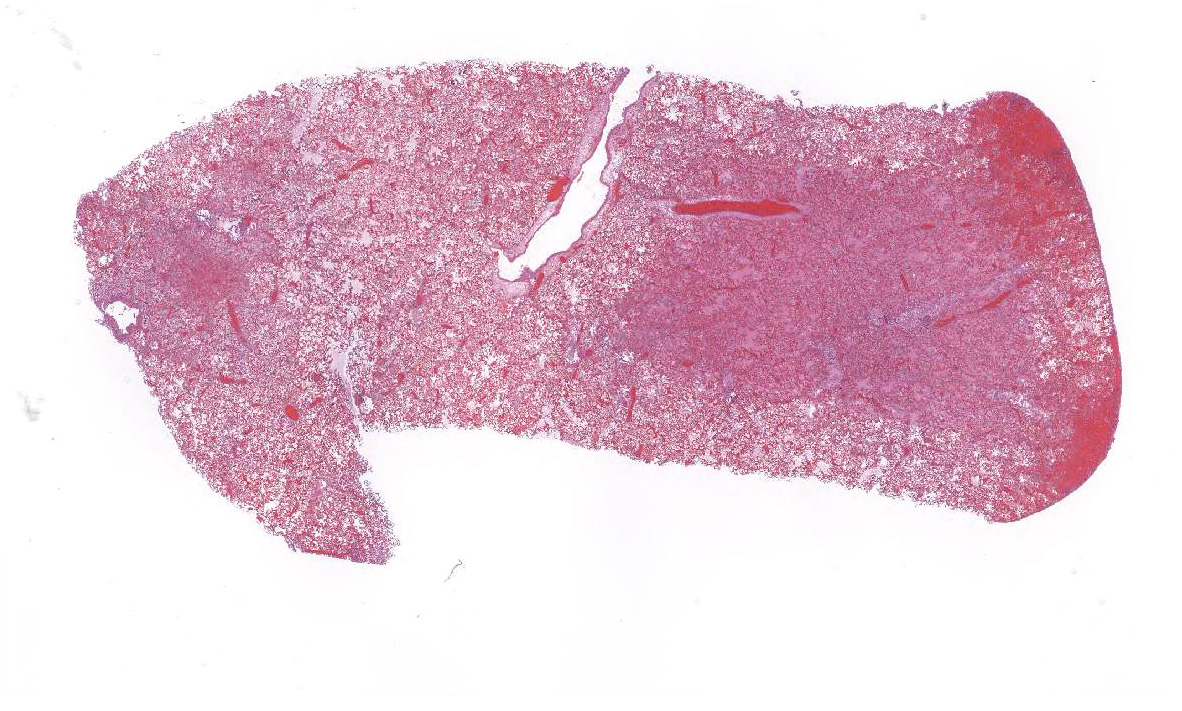
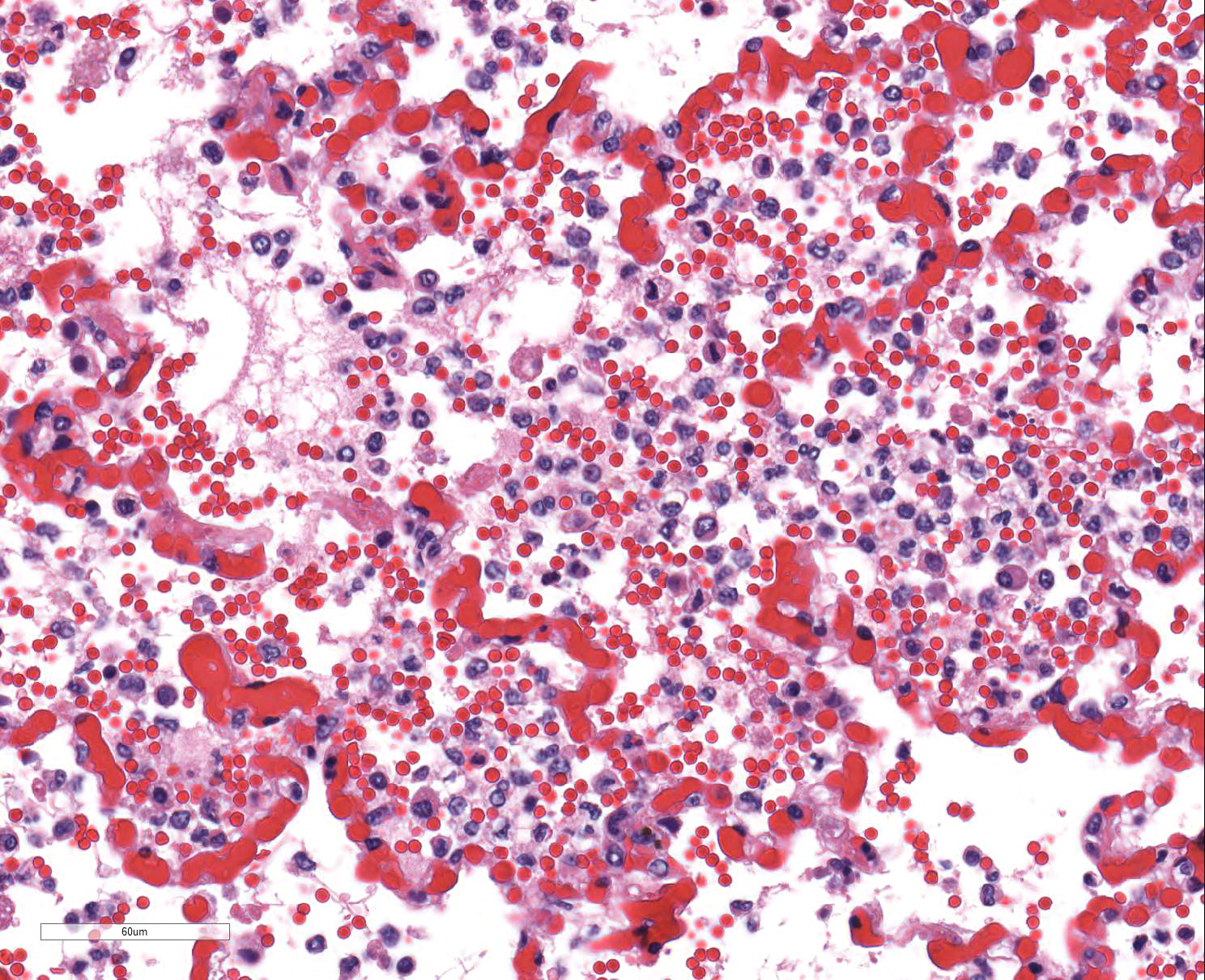
After two weeks of boarding at the referring veterinarian, the dog became acutely lethargic, tachypneic, and had two episodes of vomiting. Evaluation at the veterinary clinic that day revealed pyrexia (temperature: 106.9 oF), mild coughing, and mucoid nasal discharge. Thoracic radiographs were unremarkable. The dog was administered a fluid bolus and then later received Lasix and supplemental oxygen. Although the temperature decreased to 101.8 oF, clinical signs of dyspnea and tachypnea progressed, and the dog developed epistaxis, hemorrhagic discharge from the mouth, as well as bloody diarrhea/melena. The temperature dropped to 95.4 oF the next morning and the dog died.