WSC Conference 4, Case 4
Signalment:
3-year-old, Holstein bull (Bos taurus)
History:
A Holstein bull with a history of chronic weight loss and wasting was slaughtered on-farm for human consumption. Since multiple lesions were found in the liver, lung, bronchial and hepatic lymph nodes, the practitioner decided to submit these samples for diagnostic workup.
Gross Pathology:
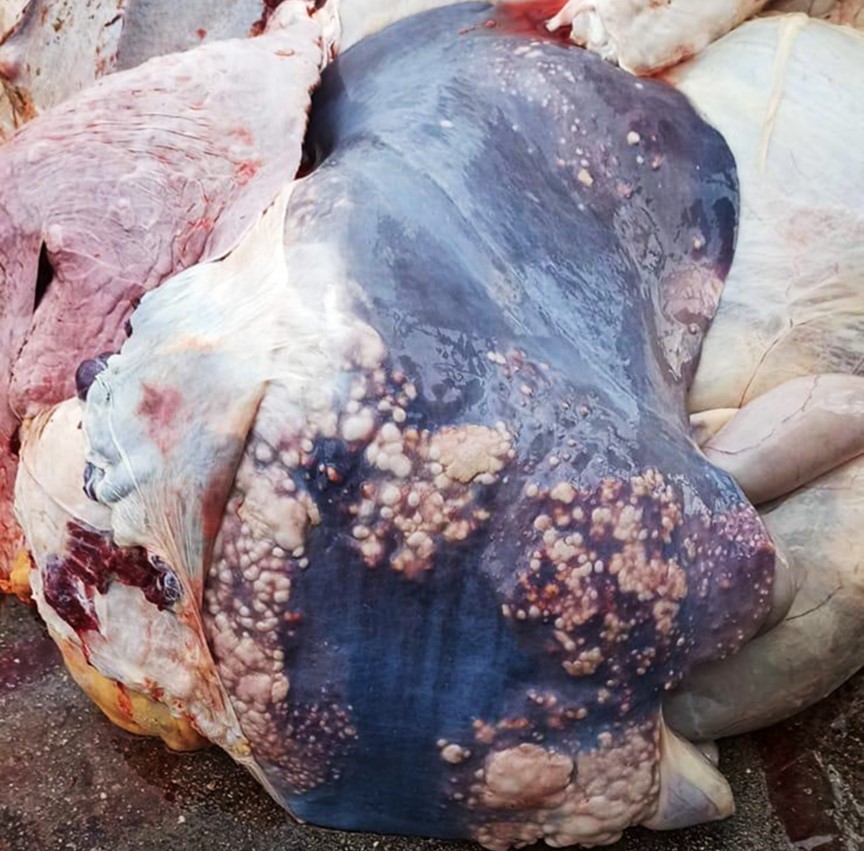
The liver, lungs, and hepatic and bronchial lymph nodes had multiple granulomas. In the liver, these granulomas were multifocal to coalescing, roughly round, nodular, firm, and were readily visible protruding from the diaphragmatic surface of the capsule. The granulomas ranged from 0.5 to 5 cm in diameter and were frequently surrounded by a pearly white capsule of connective tissue (fibrosis). On cut surface, the granulomas frequently contained a yellowish central area of caseous material (necrosis) and crepitated when sliced with the blade of the knife, suggesting mineralization.
Laboratory Results:
Mycobacterium bovis was isolated from fresh samples of lung and bronchial lymph node inoculated in egg yolk agar. Mycobacterium bovis DNA was amplified by qPCR from frozen samples of hepatic and bronchiolar lymph nodes.
Microscopic Description:
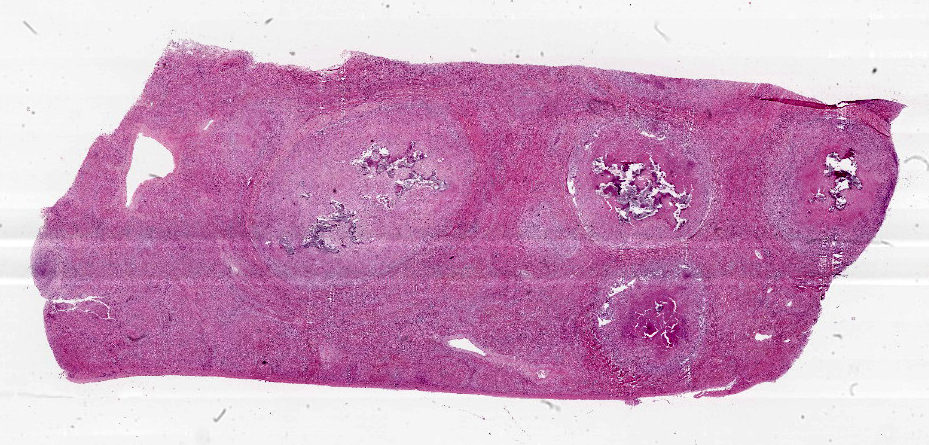
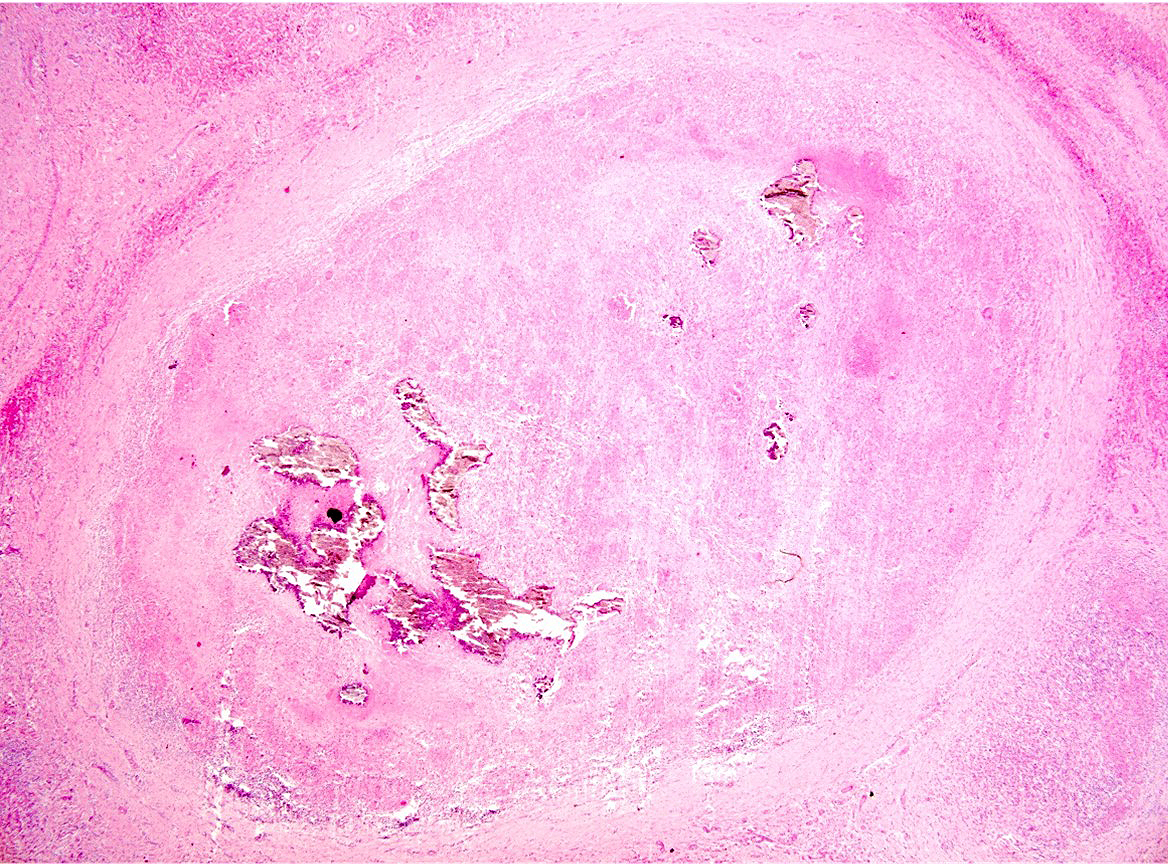
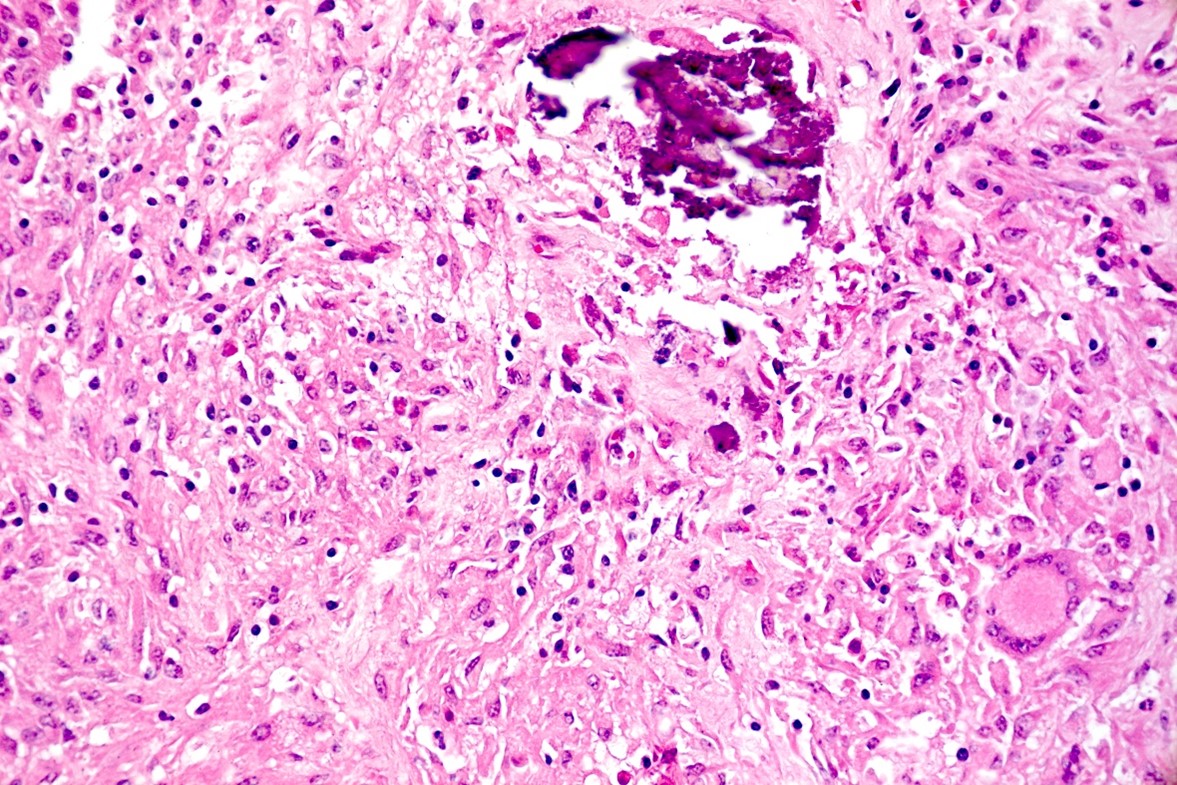
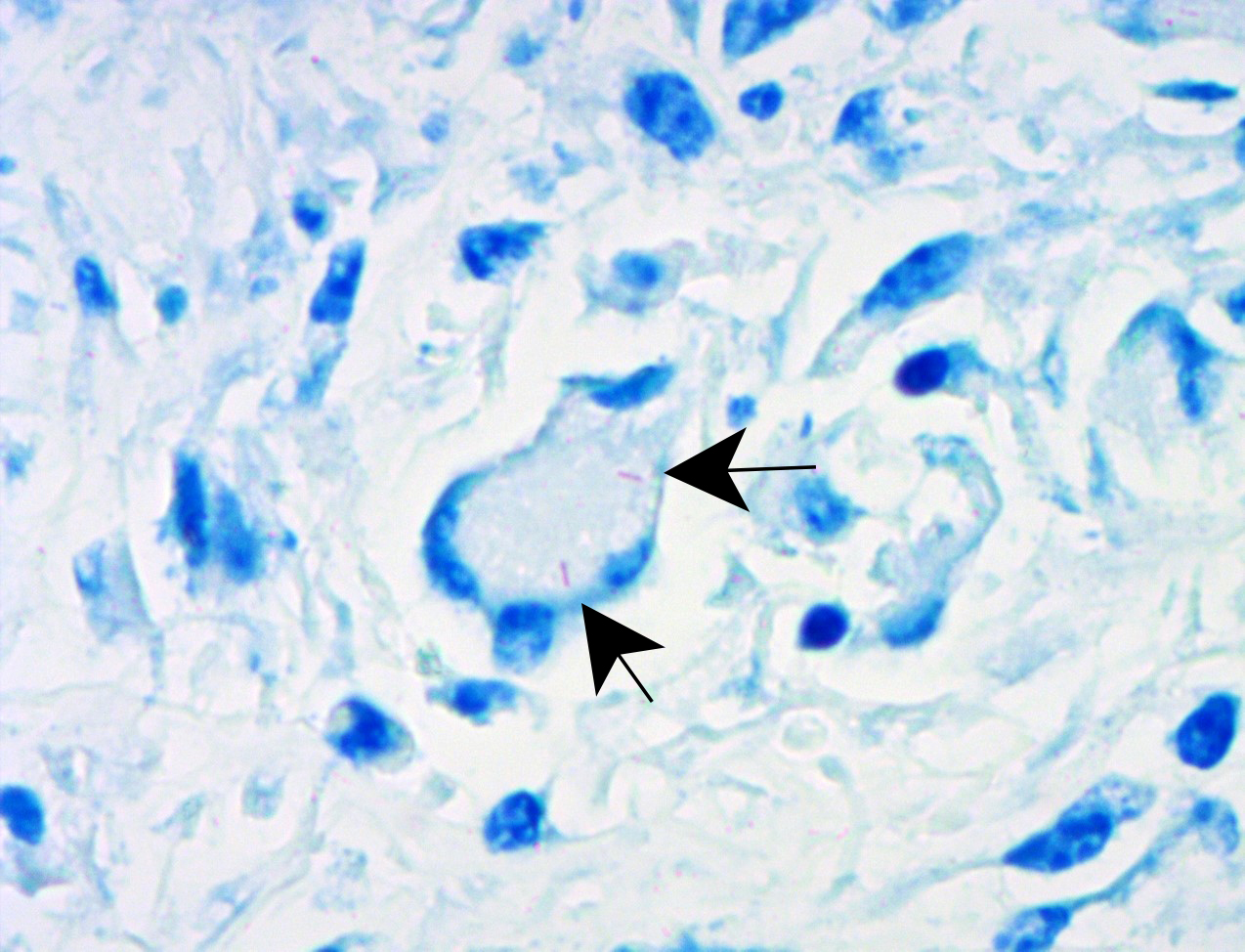
Liver: approximately 50% of the parenchyma is replaced by multiple granulomas. The granulomas are composed of a pale eosinophilic center with cellular debris (necrosis) and scattered deposits of coarse basophilic or amphophilic amorphous to crystalline material (mineralization). These necrotic and mineralized centers are surrounded and infiltrated by inflammatory cells, notably epithelioid macrophages, occasionally forming multinucleated giant cells with peripherally located nuclei (Langhans type), and fewer lymphocytes and plasma cells. The granulomas are frequently surrounded by a thick capsule of collagenous fibrous connective tissue. The hepatic parenchyma in adjacent areas is displaced and partially compressed by the granulomas. In these regions there is distortion/disruption of the hepatic cord histoarchitecture, hepatocytes are atrophic, the sinusoids are expanded by connective tissue (fibrosis), with variable degrees of portal, bridging, dissecting and perivenular fibrosis. In these areas there are also scattered infiltrates of lymphocytes, macrophages, and multinucleated giant cells. Portal areas also exhibit moderate bile duct hyperplasia. Intracellular, 2-µm long, acid fast bacilli were detected in multinucleated giant cells in histologic sections of liver stained with Ziehl-Neelsen.
Contributor’s Morphologic Diagnosis:
Liver: Hepatitis, granulomatous, multifocal to coalescing, chronic, severe with caseous necrosis and mineralization, multinucleated giant cells, intra-histiocytic acid-fast bacilli, and fibrosis, holstein bull, bovine.
Contributor’s Comment:
The diagnosis of bovine tuberculosis in this case was mainly based on pathological findings coupled with the identification of acid-fast bacilli by Ziehl-Neelsen, and isolation and molecular identification of Mycobacterium bovis.
Mycobacteria are facultative intracellular, aerobic, non-motile, pleomorphic bacilli.8,16 They resist decolorization by acids during staining procedures because of the high content of lipids of their cell walls. Hence, they are considered acid-fast bacteria and can be stained with Ziehl-Neelsen or Fite-Faraco stains, but not with Gram stains.17 The mycobacteria that cause tuberculosis are grouped within the Mycobacterium tuberculosis complex (MTBC), which includes M. tuberculosis, M. bovis, M. caprae, M. africanum, M. microti, M. canettii, M. pinnipedii, M. orygis, M. suricattae, M. mungi, and the Dassie and Chimpanzee bacilli.3,11 Within this extensive group, only two members are confirmed causes of bovine tuberculosis: M. bovis and, to a lesser extent, M. caprae.6,12
Although bovine tuberculosis has a worldwide distribution, its prevalence has declined significantly in many regions, and approximately 20 countries (mainly from Europe) are considered tuberculosis-free as a result of rigorous eradication programs.2,5 Similar eradication programs are currently being implemented in other countries, such as the US, Mexico, New Zealand, and Japan.5 The disease is still widely spread in Africa, Asia, and Central and South America.2,5
Mycobacteria are very resistant to environmental conditions, which facilitates indirect infection from contaminated sources.9 However, direct transmission from infected animals is the main route of infection.9 Inhalation of aerosols containing mycobacteria is the primary route of transmission in cattle.6,9 Mycobacterium bovis can also be excreted in feces, urine, and colostrum/milk; hence, contaminated feedstuff and water are potential sources of infection. Oral transmission requires a large infective dose.6,9 In young calves, infection through ingestion of unpasteurized colostrum/milk from infected cows is possible.9 Other uncommon infection sources and routes include cutaneous exposure, congenital infection, or transmission through semen, vaginal and uterine discharges, among others.6
Bovine tuberculosis spreads throughout the organism in two stages: i) the primary complex and ii) post-primary dissemination. After lodging in the lesion inflicted at the entry site, mycobacteria are transported to the draining lymph node(s) where they establish a secondary infectious focus, generating the primary complex.7 When both lesions (at the entry point and the local lymph node) are present, the primary complex is classified as complete, and when the lesion at the site of entry is absent (as in most cases occurring after digestive transmission) the primary complex is referred to as incomplete.7 Depending on the immune status of the host, dissemination from the primary complex (post-primary dissemination) may occur either via lymphatic or hematogenous spread, or through pre-existing anatomical routes in the organs. Each of these forms of spread will determine different post-primary presentations: late generalization or chronic organ tuberculosis.6,7 Another presentation is called early generalization, which occurs due to the infection’s spread during the early stages as a consequence of a poor immune response.7
The clinical presentation usually varies with the localization of the infection. Overall, clinical signs are nonspecific (i.e., progressive weight loss, fluctuating fever, weakness, inappetence), thus the diagnosis should not be based on clinical examination alone. It should be considered that cattle with extensive miliary lesions may be clinically normal.5,6
The characteristic gross lesion is the tubercle, a circumscribed, often encapsulated, tan to yellow granuloma, often with central caseous necrosis and/or mineralization, which has been referred to as a “caseocalcareous granuloma”.4 There is large variation in the size of the tubercles; they can be small enough to be overlooked during autopsy, or involve a major part of an organ.18 Tubercles are most frequently seen in bronchial, retropharyngeal, and mediastinal lymph nodes.4 Other sites commonly affected include the lung, liver, spleen, and the serosal surfaces of body cavities.18 Other locations, including the brain and meninges, have also been described.14
Histologically, small granulomas consist of epithelioid macrophages and few Langhans-type multinucleated giant cells and neutrophils.4,7,11 As the lesion progresses, a central area of caseous necrosis, consisting of eosinophilic homogeneous material with necrotic cell debris and mineralization develops.4,7,11 This necrotic core is surrounded by macrophages, multinucleated giant cells, lymphocytes, and occasional neutrophils.4,7,11 Over time, a fibrous connective tissue capsule is
formed. Acid-fast bacilli may be found extracellularly in the necrotic core or within the cytoplasm of macrophages and giant cells.7,11 The absence of acid-fast bacilli on histopathological examination does not rule out tuberculosis.7,18
Differential diagnoses on clinical or gross pathology grounds include many conditions such as mycobacteriosis associated with the M. avium-intracellulare complex, atypical mycobacteria, bacterial pneumonia and lung abscesses (i.e. Mycoplasma bovis, Pasteurella multocida, Trueprella pyogenes, aspiration pneumonia), actinobacillosis, fungal diseases (zygomycosis, blastomycosis, aspergillosis, histoplasmosis, coccidioidomycosis), foreign body granuloma, traumatic pericarditis, bovine leukemia virus, and cestode cysts.5,6,10 However, histologically, tuberculoid granulomas are usually easily distinguishable from all these conditions.
Diagnostic assays include direct tests for the identification of the agent, generally in postmortem specimens, such as microscopic examination, culture, immunohistochemistry, and molecular techniques.6,18 Indirect tests are performed on specimens from live animals to detect infected individuals, and can be based on the cellular (intradermal tuberculin test, gamma-interferon test, and lymphocyte proliferation test) or humoral immunity (ELISA).6.18
Contributing Institution:
Plataforma de Investigación en Salud Animal
Instituto Nacional de Investigación Agropecuaria (INIA)
La Estanzuela, Uruguay
www.inia.uy
JPC Diagnosis:
Liver: Granulomas, multifocal to coalescing, with bridging fibrosis.
JPC Comment:
As noted by the contributor, the classic lesion of tuberculosis is the tubercle, or tuberculous granuloma. Though a seemingly simple structure, the presence of a granuloma is the result of a complex sequence of inflammatory events requiring 1) the presence of an inciting agent with indigestible, poorly degradable, and persistent antigens, 2) a Th1 host immune response, and 3) an interplay between cytokines, chemokines, and other inflammatory mediators within the chronic inflammatory lesion.1
Granulomas are classically described as forming via a Type IV, cell-mediated hypersensitivity reaction. Sensitization occurs upon initial exposure to the antigen, which forms antigen-specific T memory lymphocytes. With chronic exposure, here enabled by the virulence factors that allow Mycobacterium bovis to live within macrophages indefinitely, memory T lymphocytes recognize these persistent antigens presented in MHC II complexes by macrophages, and initiate a complex inflammatory sequence. Th1 lymphocytes secrete cytokines, such as TNF and IL-17, which recruit monocytes from the circulation to the site of inflammation. Once in the tissue, Th1-lymphocyte derived IFN-? converts the monocytes to activated macrophages. The activated macrophages then secrete cytokines such as IL-12 which facilitate further development of Th1 lymphocytes.15 Activated macrophages also elaborate IL-1 and TNF-α, both of which act to increase local expression of adhesion molecules on endothelial cells, thus recruiting more leukocytes to the inflammatory party. Meanwhile, Th1 lymphocytes secrete IL-2, which acts in an autocrine fashion to induce Th1 lymphocyte proliferation.15
The result of this persistent, Th1 lymphocyte-driven inflammation is the granuloma, which functions to wall off persistent antigenic stimuli from the rest of the body and is typically described as paucibacillary due to the few identifiable acid-fast bacteria. There are only a few conditions that are associated with the formation of granulomas, so their presence provides the astute pathologist with a radically pruned differential list.
In contrast to this nodular granulomatous morphology, certain mycobacterial conditions are associated with diffuse/lepromatous inflammation which is paradigmatically associated with a Th2 lymphocyte-predominant response. Lepromatous responses are poorly delineated, have a widespread distribution, abundant bacteria, relatively fewer lymphocytes, many macrophages, and lack a distinct capsule.1
In lepromatous immunological response, Th2 lymphocytes elaborate IL-4, IL-10, and TGF-β which inhibit the Th1 response and inactivate the microbicidal responses of macrophages, most notably iNOS. This facilitates the survival of the bacilli and shuts down the interplay between lymphocytes and macrophages that lead to granuloma formation.13 The resulting diffuse granulomatous inflammation is seen in Mycobacterium leprae infection in humans and Mycobacterium avium subsp. paratuberculosis infection (Johne’s disease) in cattle, sheep, and goats.1
This case rounds out this week’s tour of classic entities with excellent examples of paradigmatic, Th1-driven tubercle formation. Conference participants felt the tubercles were so paradigmatic that a morphologic diagnosis of “granulomas” encapsulated a host of implied histologic features, including granulomatous inflammation, mineralization, necrosis, and concentric fibrosis. Conference participants discussed the striking bridging fibrosis and thought the consequent ischemia could account for the diffuse hepatocellular atrophy observed in less affected areas of the hepatic parenchyma.
References:
- Ackermann MR. Inflammation and healing. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier;2022:143-145.
- Awada L, Tizzani P, Erlacher-Vindel E, Forcella S, Caceres P. Bovine tuberculosis: worldwide picture. In: Bovine tuberculosis. Wallingford, Oxfordshire, UK: CABI; 2018:1–15.
- Azé J, Sola C, Zhang J, et al. Genomics and machine learning for taxonomy consensus: the mycobacterium tuberculosis complex paradigm. PLoS One. 2015;10 (7):e0130912.
- Caswell JL, Williams KJ. Respiratory System. In: Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier;2016:465-591.
- Center for Food Security & Public Health (CFSPH). Zoonotic tuberculosis in mammals, including bovine and caprine tuberculosis. 2019.
- Constable P, Hinchcliff K, Done S, Grünberg W. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed. Saunders Elsevier;2017:2015–2023.
- Domingo M, Vidal E, Marco A. Pathology of bovine tuberculosis. Res Vet Sci. 2014;97(Supplement):S20–S29.
- Kanipe C, Palmer MV. Mycobacterium bovis and you: A comprehensive look at the bacteria, its similarities to Mycobacterium tuberculosis, and its relationship with human disease. Tuberculosis (Edinb). 2020;125:1–28.
- Menzies F, Neill S. Cattle-to-cattle transmission of bovine tuberculosis. Vet J. 2000;160:92–106.
- Ortega J, Uzal FA, Walker R, et al. Zygomycotic lymphadenitis in slaughtered feedlot cattle. Vet Pathol. 2010;47(1): 108–115.
- Palmer MV, Kanipe C, Boggiatto PM. The bovine tuberculoid granuloma. Pathogens. 2022;11(1).
- Pesciaroli M, Alvarez J, Boniotti MB, et al. Tuberculosis in domestic animal species. Res Vet Sci. 2014;97(S):S78–S85.
- Rodrigues de Sousa J, Sotto MN, and Quaresma JAS. Leprosy as a complex infection: breakdown of the Th1 and Th2 immune paradigm in the immunopathogenesis of disease. Front Immunol. 2017;8:1635.
- Silveira AM, Nascimento EM, Konradt G, et al. Tuberculosis of the central nervous system in cattle in Paraíba, Brazil. Pesqui Vet Bras. 2018;38(11):2099–2108.
- Snyder P. Diseases of Immunity. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier; 2022:322-325.
- Ünüvar S. Microbial foodborne diseases. Foodborne Dis. 2018;15:1–31.
- Vilchèze C, Kremer L. Acid-fast positive and acid-fast negative mycobacterium tuberculosis: the Koch paradox. Microbiol Spectr. 2017;5(2).
- World Organisation for Animal Health (OIE). Bovine tuberculosis. In: OIE Terrestrial manual. 8th ed. OIE;2018;1058-1074.