Signalment:
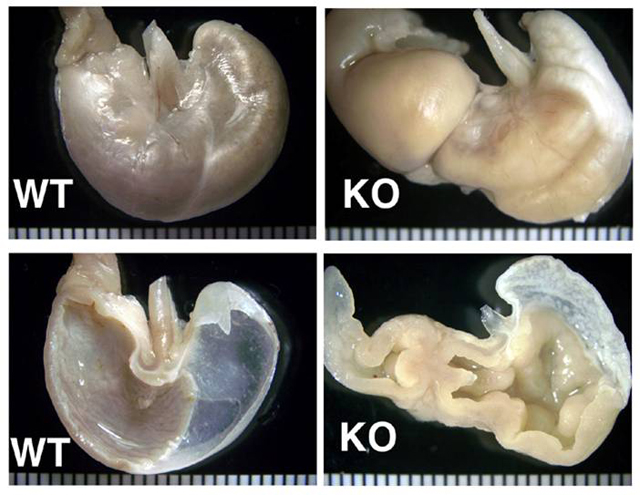
Gross Description:
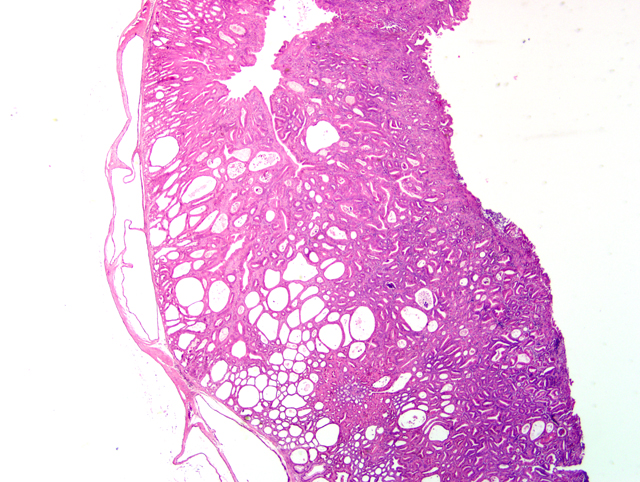
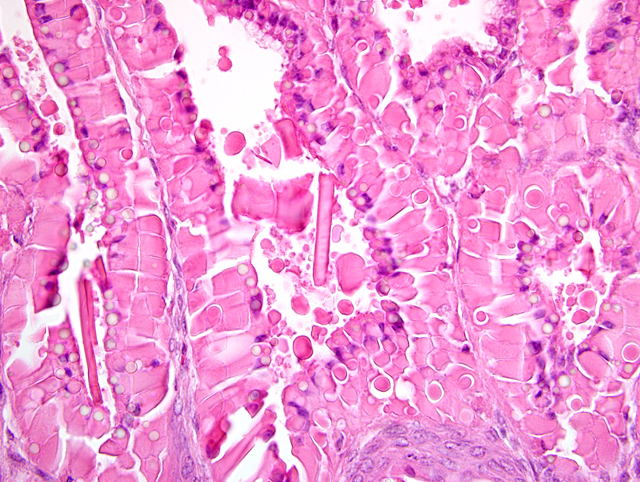
Histopathologic Description:
Morphologic Diagnosis:
1. Stomach (glandular portion): Severe diffuse adenomatous hyperplasia with hyalinosis, mild to moderate multifocal mononuclear, eosinophilic and erosive gastritis.
2. Stomach (squamous portion): Moderate diffuse orthokeratotic hyperkeratosis.
3. Proximal duodenum: Mucosal metaplasia with hyalinosis, moderate to marked diffuse chronic-active lymphoplasmacytic and eosinophilic enteritis.
Condition:
Contributor Comment:
     i. As a spontaneous lesion in several mouse strains including Han:NMRI,(14) strain I,(1) aging C57BL/6x129 and 129/Sv mice(2) and CD-1 mice.(3)
     ii. In experimental infection with Helicobacter felis of WT C57BL/6 mice(5) and several mutant strains (e.g. IL-10-/-).
     iii. With long-term treatment of mice with H2 blocking drugs (13) and other antisecretory compounds.(1)
     iv. In experimental infection of immunodeficient mice with Taenia taeniaformis.(8)
     v. In several knockout and transgenic mice.(10)
     vi. In densely housed mice.(7)
     vii. In association with colitis in mutant mice.(4,5)
The gastric hyperplasia observed in the above conditions may have distinguishing gross and/or histologic features depending on the specific etiology. Common to these is mucosal hyperplasia with loss of specialized cells, also referred to as mucous metaplasia. The degree of inflammatory infiltration is variable. In the gastric lesion from this knockout, the degree of hyperplasia clearly outstrips inflammation. Alcian blue and periodic acid Schiff stains demonstrate altered mucin production, as expected.(7) The extent of hyalinosis in this case is striking. The globular hypereosinophilic material was strongly immunoreactive with a polyclonal antibody raised against Ym1/T-lymphocyte-derived eosinophil chemotactic factor (immunohistochemical staining kindly provided by JM Ward, NCI). Proteins in this family are believed to play a role in mucosal defense against parasites.(15)
The cause of the lesion in this knockout, as in many of the above, remains undetermined. In some cases it may be due to hormonal imbalance, e.g. hypergastrinemia. The hyperkeratosis in the forestomach is consistent with insufficient mechanical abrasion of keratin due to reduced food intake.(3) The proliferative enteritis in the proximal duodenum is always encountered in conjunction with the gastric lesion, to which it is assumed to be secondary. While in man metaplasia of the gastric mucosa is associated with an increased risk of carcinoma, the murine condition is often not associated with increased incidence of gastric malignancy.(3,14)
In domestic animals, conditions which bear some resemblance to this lesion include giant hypertrophic gastritis (Menetrier's disease) in dogs and chronic abomasitis with mucous metaplasia in ruminants caused by infestation with Ostertagia sp. ("Morocco leather").
JPC Diagnosis:
1. Stomach, glandular: Hyperplasia, adenomatous, diffuse, marked, with cystic mural herniation, chief and parietal cell loss, epithelial hyalinosis, mucous metaplasia acicular eosinophilic crystalline material, and mild multifocal eosinophilic and neutrophilic gastritis.Â
2. Stomach, squamous: Hyperkeratosis, orthokeratotic, diffuse, moderate.
Conference Comment:
During the conference, participants compared the slide for this case with a section of normal mouse stomach to emphasize the striking mucous metaplasia and hyalinosis present herein. Hyalinosis has been described in a variety of tissues and a variety of mouse strains. In the glandular stomach of affected 129S4/SvJae and B6;129 mice, the extracellular and eosinophilic crystals were determined to be composed of Ym2 protein, a chitinase-related protein. Other sites reportedly affected by hyalinosis in mice include biliary and pancreatic duct epithelium, and the respiratory epithelium lining the nasal cavity and respiratory tract.(15)
In addition to the examples cited by the contributor, conference participants discussed other instances of proliferative gastric lesions in domestic animals, including equine hypertrophic gastritis associated with Habronema spp. and Trichostrongylus axei, hypertrophic gastritis in nonhuman primates caused by Nochtia nocti, gastritis in pigs caused by Hyostrongylus rubidus, and gastritis in cats caused by Ollulanus tricuspis.(6) In addition to ostertagiosis, Cryptosporidium andersoni has been recognized as a cause of proliferative abomasitis in cattle.(11)
References:
2. Ennulat D, Kawabe M, Kudo G, Peters JM, Kimura S, Morishima H, Gonzalez FJ, Ward JM: Hyperplastic gastric mucosal lesions with eosinophilic cytoplasmic inclusions in aging C57BL/6N x 129 and Sv/129 mice. Abstract Vet Path 35:456, 1998
3. Faccini JM, Abbott DP, Paulus GJJ: Mouse Histopathology: a Glossary for Use in Toxicity and Carcinogenicity Studies, pp. 98-102. Elsevier, New York, NY, 1990
4. Fernandez-Salgureo PM, Ward JM, Sundberg JP, Gonzalez FJ: Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 34:605-614, 1997
5. Fox JG, Dangler CA, Schauer DB: Inflammatory bowel disease in mouse models: role of microintestinal microbiota as proinflammatory modulators. In: Pathology of Genetically Engineered Mice, eds. Ward JM, Mahler JF, Maronpot RR, Sundberg JP, pp. 302-303. Iowa State University Press, Ames, IA, 2000
6. Gelberg HB: Alimentary system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 339-341. Mosby Elsevier, St. Louis, MO, 2007
7. Greaves P, Boiziau JL: Altered patterns of mucin secretion in gastric hyperplasia in mice. Vet Pathol 21:224-228, 1984
8. Lagapa JT, Konno K, Oku Y, Nonka N, Ito M, Kamiya M: Gastric hyperplasia and parietal cell loss in Taenia taeniaformis inoculated immunodeficient mice. Parasito Int 51:81-89, 2002
9. Leininger JR, Jokinen MP, Dangler CA, Whiteley LO: Oral cavity, esophagus and stomach. In: Pathology of the mouse, eds. Maronpot RR, Boorman GA, Gaul BW, 1st ed., pp. 36-37. Cache River Press, Vienna, IL, 1999
10. Maehler M, Rozell B, Mahler JF, Merlino G, Devor-Henneman DE, Ward JM, Sundberg JP: Pathology of the gastrointestinal tract of genetically engineered and spontaneous mutant mice. In: Pathology of Genetically Engineered Mice, eds. Ward JM, Mahler JF, Maronpot RR, Sundberg JP, pp. 269-298. Iowa State University Press, Ames, IA, 2000
11. Masuno K, Yanai T, Hirata A, Yonemaru K, Sakai H, Satoh M, Masegi T, Nakai Y: Morphological and immunohistochemical features of Cryptosporidium andersoni in cattle. Vet Pathol 43:202-207, 2006
12. Park JH, Seok SH, Baek MW, Lee HY, Kim DJ, Park JH: Gastric lesions and immune responses caused by long-term infection with Helicobacter heilmannii in C57BL/6 mice. J Comp Pathol 139:208-217, 2008
13. Poytner D, Selway SAM, Papworth SA, Riches SR: Changes in the gastric mucosa of the mouse associated with long lasting unsurmountable histamine H2 blockade. Gut 27:1338-1346, 1986
14. Rehm S, Sommer R, Derberg F: Spontaneous nonneoplastic gastric lesion in female Han:NMRI mice and influence of food restriction throughout life. Vet Pathol 24:216-225, 1987
15. Ward, JM, Yoon M, Anver M, Haines DC, Kudo G, Gonzalez FJ, Kimura S: Hyalinosis in Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B2,129 mice. Am J Pathol 158:323-332, 2001