WSC 2024 Conference 13, Case 2
Signalment:
3-day-old male White Shorthorn calf (Bos taurus)
History:
A male, White Shorthorn calf was delivered with assistance and born blind. This was the third calf to be born blind in the last two calving seasons. All blind calves were sired by the same White Shorthorn bull and offspring from other sires were unaffected.
Initially, the submitting veterinary surgeon suspected Vitamin A deficiency during gestation. However, a bolus strategy containing Vitamin A had no effect. Also, cohort sampling of 10 cows revealed unremarkable vitamin A levels in 9/10 cows and, therefore, the herd relevance of Vitamin A deficiency was unclear. The herd is vaccinated against BVD and all animals have tested negative for BVD.
Clinical examination of the blind calf by veterinary ophthalmologists revealed bilaterally microphthalmic globes, a wandering nystagmus, and a tendency for ventral rotation. Both pupils were small and the pupillary light reflexes and dazzle reflexes were absent. Close examination of both eyes revealed anterior segment dysgenesis with microcornea, shallow anterior chambers and small pupils. Detailed assessment of the lenses was not feasible due to small pupil size. The calf was euthanized by barbiturate injection.
Gross Pathology:
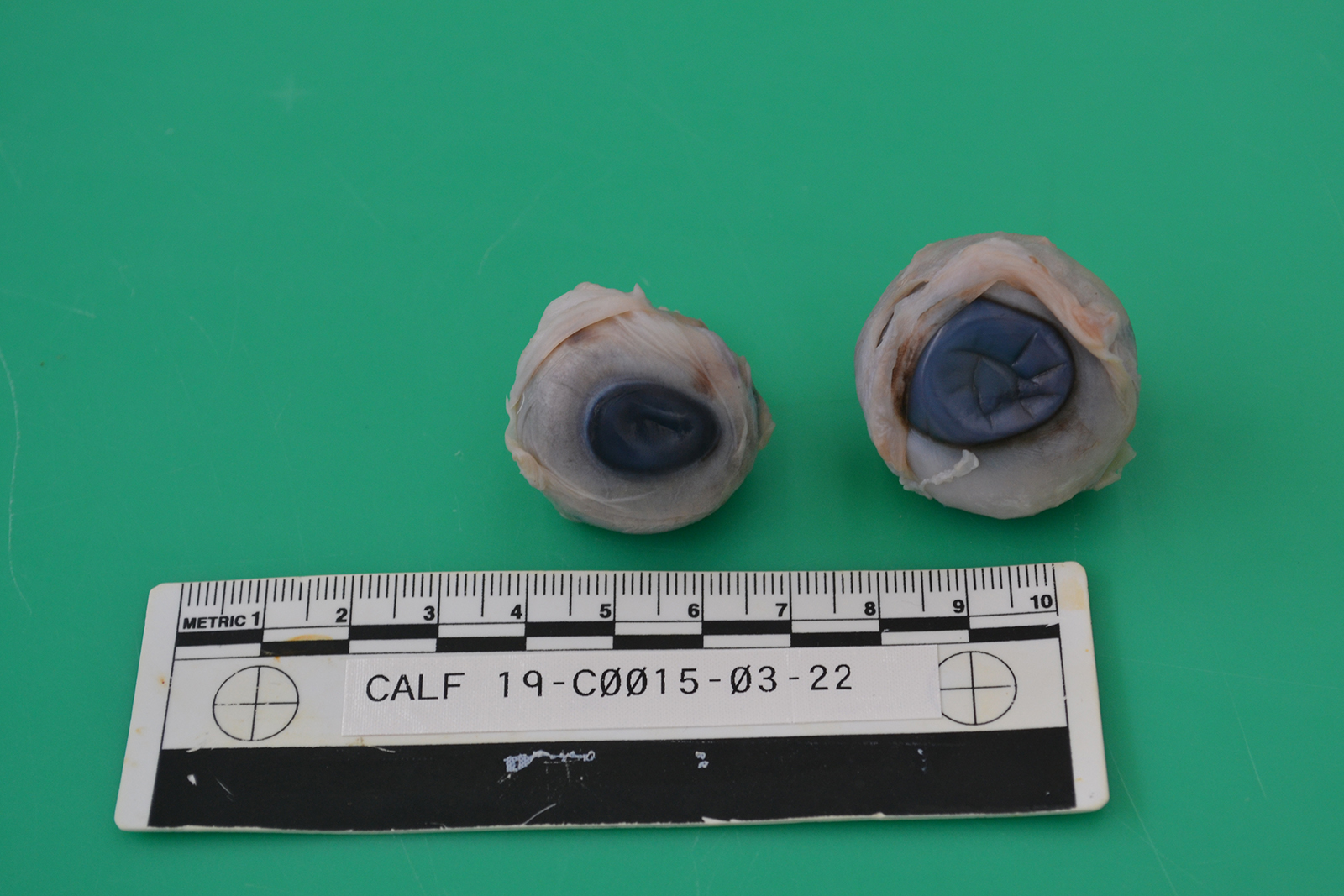
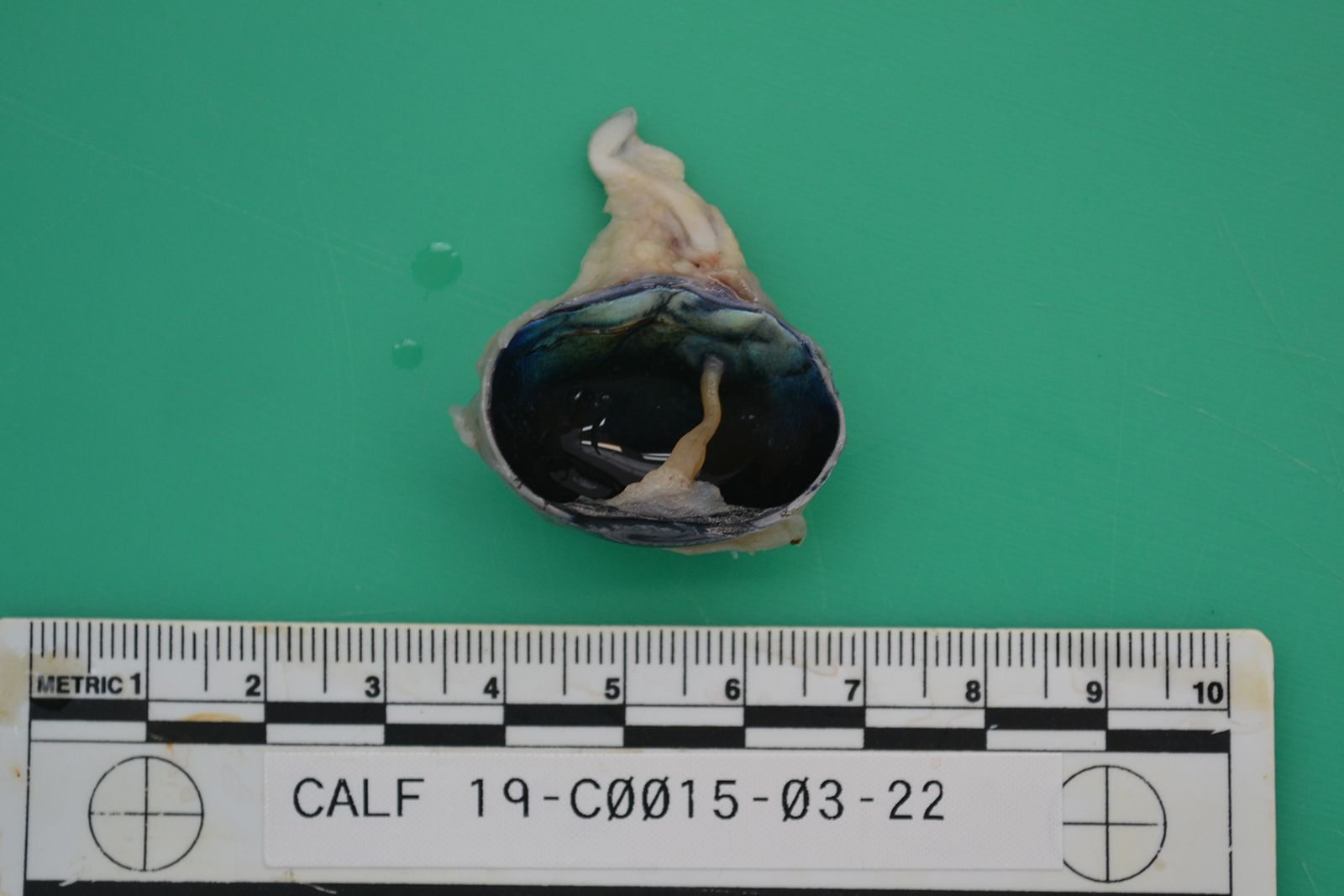
The ocular globes are mildly sunken into the orbits. Both globes are reduced in diameter, measuring 2.7 x 2.5 cm on the right (OD) and 3.1 x 2.7 cm on the left (OS), respectively. Bilaterally, there are multifocal scleral haemorrhages. Examination of the cut-sections post-fixation reveals both eyes have little obvious lens remaining. The left eye shows collapse of the anterior chamber and there are presumptive lens remnants. These are connected by a cream colored, soft membrane to the posterior pole of the eye. The other organs, including the cerebrum, cerebellum and brainstem were grossly unremarkable.
Laboratory Results:
BVDV PCR (spleen): Negative.
Microscopic Description:
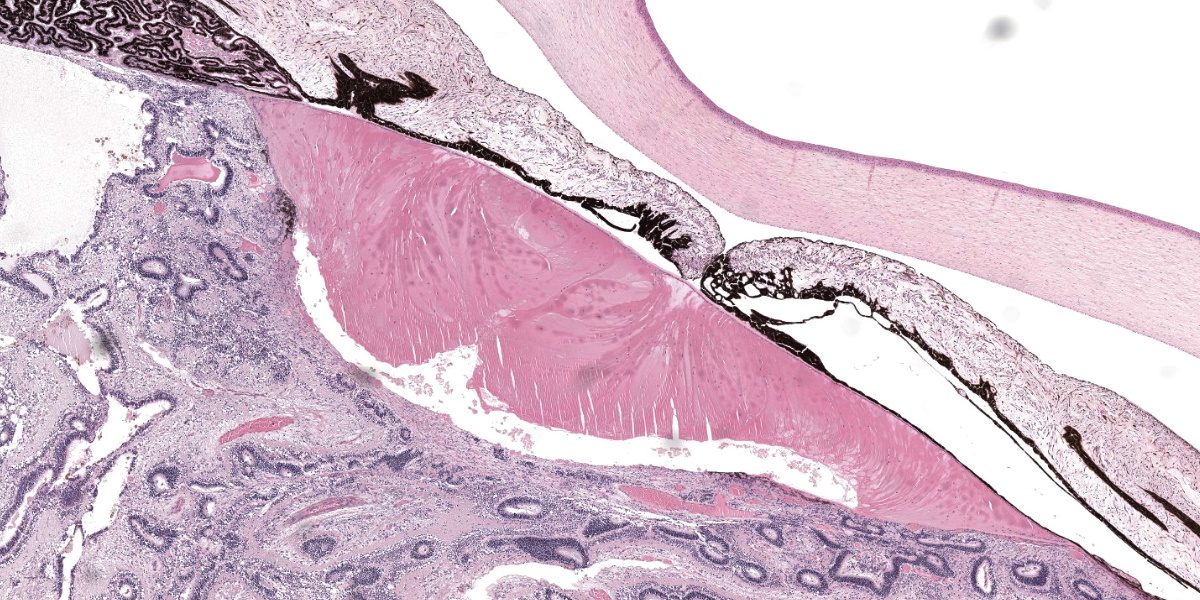
As both eyes showed similar macroscopic and histopathological changes, only the left globe is described below.
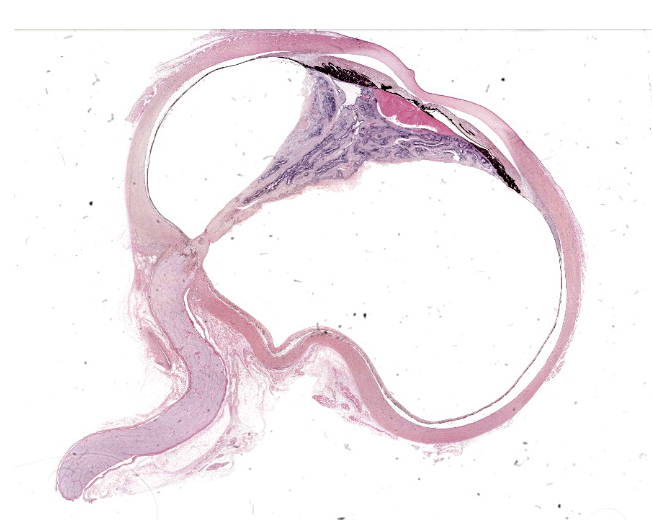
Eye (OS): Compressing against the posterior aspect of the ciliary body and the posterior pole of the lens and displacing these structures anteriorly, there is a diffusely detached, disorganized and folded retina, which crosses the posterior chamber and joins with the optic nerve head. The underlying retinal pigmented epithelium is multifocally rounded and hypertrophied (tombstoning).
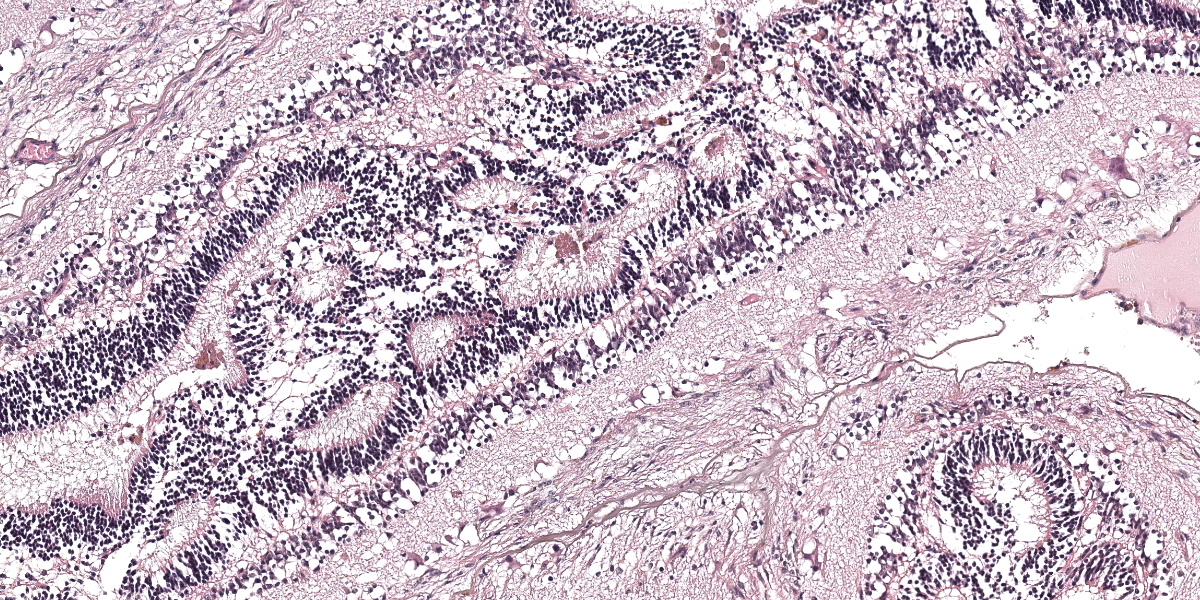
The dysplastic retina is characterized by the following histological features: multifocally the outer nuclear layer forms numerous tubular structures and rosettes surrounding a central space containing eosinophilic fibrils (presumptive necrotic rods and cones) admixed with scattered degenerate neutrophils. The outer plexiform layer surrounding these rosettes and tubules is diffusely thinned and the inner and outer nuclear layers frequently coalesce. The inner plexiform layer, ganglion cell layer, nerve fiber layer and internal limiting membrane multifocally frequently blend together and are not readily distinguishable. However, the ganglion cell layer can be occasionally distinguished in areas of retinal folding. Multifocally, randomly distributed throughout the dysplastic retina there are large lakes of amorphous eosinophilic material admixed with melanocytes and karyorrhectic cellular debris (lytic necrosis). The nerve fibers within the adjacent optic nerve head are mildly vacuolated and there is a diffusely, mildly increased cellularity (likely oligodendrocytes) within the endoneurium of the optic nerve. As a result of the aforementioned changes, the anterior chamber is markedly collapsed, and the iris and ciliary body attach to the anterior pole of the lens (posterior synechiae).
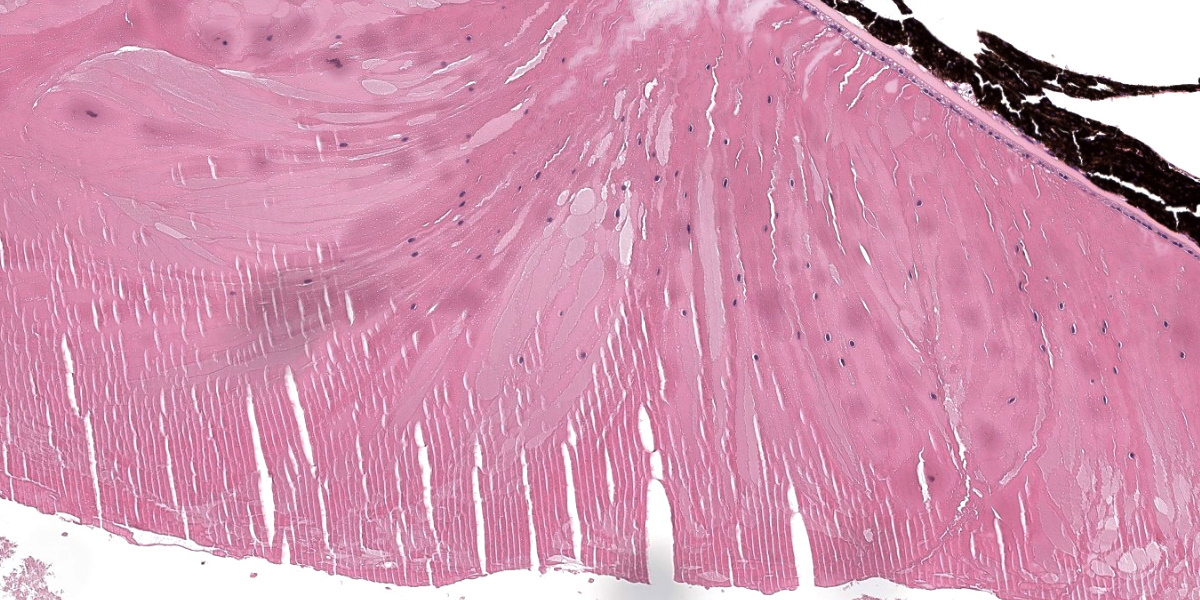
The lens shows one or more of the following histological features: markedly reduced size, liquefaction of the cortical and nuclear fibers and moderate numbers of swollen cells with scattered retained nuclei (bladder cells). The posterior contour of the lens is uneven and there is rupture of the posterior capsule with formation of multifocal Morgagnian globules (cataract). Fragments of ruptured lens stroma are frequently surrounded by the previously described dysplastic retina. The corneal stroma is multifocally mildly edematous.
Contributor’s Morphologic Diagnosis:
Globe (OS): Severe retinal dysplasia with posterior synechiae and secondary cataract.
Contributor’s Comment:
Retinal dysplasia is an anomaly of the neuroectoderm that can be defined as abnormal differentiation of the retina characterized by glial proliferation and disorganization of the retinal layers.11 As ruminants are born with mature retinas, unlike dogs and cats which continue to have post-partum retinal development, the underlying etiological cause for the retinal dysplasia in ruminants occurs in utero. In cattle and ruminants, common etiological causes include: viral infection (e.g., BVDV or bluetongue virus), hypovitaminosis A, and genetic causes such as those documented in Shorthorn calves.1,3,6,10,11
In cattle, post-necrotic retinal scarring of the developing retina associated with infection by BVDV between 79 and 150 days of gestation is the most frequently studied retinal dysplasia of viral aetiology.11 The most significant findings suggesting a viral versus a genetic cause are the presence of inflammatory infiltrates, post-necrotic retinal scarring, and scarring within the optic nerve and the choroid. BVDV has an affinity for neural tissues and, therefore, all calves with retinal dysplasia induced by the virus should also have cerebellar hypoplasia with or without hydrocephalus or hydranencephaly. While areas of necrosis are seen within the retina in this case, there was no evidence of retinal scarring or cerebellar hypoplasia, hydrocephalus, hydranencephaly or inflammation and the calf had a negative result from a BVDV PCR, making this differential less likely.
Bluetongue virus has only been described in the literature as causing retinal dysplasia in sheep, and the United Kingdom is currently free from the disease, with the last outbreak occurring in 2007.1
Retinal dysplasia has been described in some cases of hypovitaminosis A. This deficiency has been linked to feeding of brewer’s grain, sorghum, or wheat straw which all contain low β-carotene activity and low potential vitamin A activity.10 However, in cases of hypovitaminosis A there are usually additional changes, including a reduction in the size of the optic nerve, which were not observed in this case. Furthermore, the clinical history in this case of incidences being limited to calves from one sire and the non-responsiveness to Vitamin A supplementation the following calving season make involvement of hypovitaminosis A very unlikely.
To conclude, the most likely cause of the retinal dysplasia in this calf is a ‘true’ retinal dysplasia with an underlying genetic etiology, especially as in this case only calves from the same sire showed similar ocular changes. The previously documented cases of retinal dysplasia in Shorthorn cattle demonstrated a concurrent hereditary internal hydrocephalus which was not grossly observed in the submitted case. A recessive or incompletely penetrant dominant mode of inheritance was suggested, but the exact genetic mutation in Shorthorn cattle has not been identified.3 Similarly, a genetic retinal dysplasia in Hereford calves has been described with a proposed dominant inheritance of varied expressivity although again, the underlying genetic mutation has not been defined.5 Finally, other breeds of cattle that have suspected congenital retinal dysplasias documented in the literature include Simmentals and Japanese Black Cattle.8,9
The pathogenesis of retinal dysplasia in cattle is not as well characterized as in other domestic species, such as dogs, but is expected to follow similar mechanisms which are described below.11
Primary (type 1) or true retinal dysplasia is rare. It occurs due to failure of maturation by an inherently defective retinal pigmented epithelium (RPE) or failure of apposition of the two layers of the optic cup. The condition is histologically characterized by retinal rosettes, retinal folds, blending of the nuclear layers, glial scars, loss of retinal cells and retinal detachment. Rosettes should be composed of a central lumen encompassed by 1-3 layers of neuroblasts.
Secondary (type 2), or post-necrotic retinal dysplasia, is common in cattle. Secondary retinal dysplasia occurs when an infectious cause (most likely viral-induced) triggers an initial nonsuppurative panuveitis and retinitis with an associated multifocal retinal and choroidal necrosis. Over several weeks the inflammation reduces and there are scant remnants of inflammatory cells remaining microscopically and only post-necrotic scarring. The condition is characterized histologically by the presence of residual inflammation and post-necrotic retinal scarring, optic nerve scarring, and choroidal scarring. The RPE undergoes reactive hyperplasia, migration into the scarred retina or forms metaplastic, multilayered fibroglial plaques to replace the simple cuboidal epithelium. The nuclear layers are disorganized and there is rosette formation. Lesions are more common bilaterally in the non-tapetal retina and are often asymmetrical.
Retinal folding is a type of retinal dysplasia that is common in dogs and is caused by an unbalanced growth rate between the retina and the outer layers of the optic cup (choroid and sclera) or defective RPE signaling. Histologic examination reveals infolding of the neuroblastic layer away from the RPE. The folds may be transient and disappear with continued choroidal and scleral growth.
|
Species |
Cause |
|
Sheep |
Bluetongue virus |
|
Dogs |
Adenovirus, herpesvirus, genetically inherited breed-related dysplasia (English Springer Spaniel, Collies, Miniature Schnauzers, Labrador Retrievers, Samoyeds, Dobermans, Akitas, Chow Chows, Australian Shepherd dogs).7 |
|
Cats |
Feline leukemia virus, feline panleukopenia virus, genetically inherited breed-related dysplasia (Abyssinian). |
|
Chickens |
A genetic retinal dysplasia linked to a mutation in the MPDZ gene has been identified.4 |
|
Table 1-1: Selected causes of retinal dysplasia. |
|
Contributing Institution:
Department of Pathobiology and Population Sciences (PPS)
Royal Veterinary College
Brookmans Park, Hatfield, Hertfordshire
www.rvc.ac.uk
JPC Diagnosis:
Eye: Ocular dysgenesis with retinal dysplasia, retinolenticular adhesion, and cataract.
JPC Comment:
The contributor gives an excellent overview of the pathogenesis, classification, and histopathologic lesions of retinal dysplasia. The various manifestations of retinal dysplasia can best be understood in the context of ocular organogenesis, which begins with bilateral evaginations of the forebrain that become separated from the developing diencephalon by the optic stalks.11 These evaginations, called optic vesicles, grow outward toward the surface ectoderm, where signaling from a thickened area of surface ectoderm called the lens placode causes the distal optic vesicles to evaginate. These evaginations form the bilayered optic cups, and signaling from and to the lens placode causes its evagination and separation from the ectoderm to form the developing lens.12 The inner layer of the optic cup proliferates and becomes the retinal neuroepithelium, while the outer layer of the optic cup, via interactions with the surrounding mesenchyme, becomes the retinal pigmented epithelium (RPE). The surrounding mesenchyme subsequently develops into the vascular and fibrous tunics of the eye.11,12
The RPE is distinguished from the neural retina by the presence of pigment and by a monolayer epithelial arrangement as opposed to the pseudostratified epithelial arrangement of the neural retina. If these features are not present, this is considered a failure of RPE differentiation, which has implications for further ocular development as the RPE is required for subsequent retinal and ocular morphogenesis.13 In early development, the neural retina and the RPE depend on intercellular cross-talk for normal development, and hyperplasia of one layer can occur at the expense of the other if this complex signaling is perturbed.13
As noted by the contributor, true retinal dysplasia is caused either by improper apposition of the two layers of the optic cup, leading to improper cell-cell signaling interactions during development, or to an improperly formed or defective RPE.11 The histologic hallmark of true retinal dysplasia is the rosette, several most excellent examples of which are present in the examined slide, composed of a central lumen surrounded by 1-3 layers of neuroblasts with varying amounts of retinal differentiation and pink fibrils, representing photoreceptors, projecting into the lumen.11
In dogs, true retinal dysplasia occurs in combination with chondrodysplasia in several dog breeds, including the Labrador Retriever and Samoyeds, and retinal dysplasia may be accompanied by persistent hyaloid membranes and cataracts in these breeds.11
Conference participants quickly found themselves engaged in discussions at once semantic and existential. Several participants interpreted the lenticular changes and the attendant inflammation as phacoclastic uveitis; however, Dr. Neto felt the term inappapropriate given that it implies a lens capsule rupture. In this case, the lens, including the lens capsule, likely formed neither correctly nor completely. Can that which was never formed be ruptured? Similarly, the term “retinal detachment” implies that the retina was once attached to the RPE, perhaps an erroneous assumption in the context of retinal dysplasia, where the layers of the optic cup were likely never properly opposed. Can that which was never together be separated?
More practically, Dr. Neto noted that this case is a great example of why one should always evaluate the slide grossly prior to histologic examination. Doing so in this case reveals an entire calf eye that fits on a standard slide – a likely indication that the eye is too small. Dr. Neto then discussed the difference between nanophthalmos, where the eye is smaller than the control eye, but is anatomically intact, and microphthalmos. Microphthalmos comes in two varieties. In simple microphthalmos, the eye is small due to a developmental problem with the optic vesicle or due to difficulty maintaining intraocular pressures, but the eye is anatomically intact. Some consider simple microphthalmos and nanophthalmos to be equivalent conditions. Complex microphthalmos occurs when, as in this case, the eye is small but is anatomically abnormal, often with disorganized tunics. Microphthalmos can encompass anterior segment abnormalities such as cataract, as well as posterior segment abnormalities such as chorioretinal coloboma and retinal dysplasia.
Discussion of the morphologic diagnosis reflected the philosophical discussion noted above, with participants choosing to describe the retinal changes as simple dysplasia versus detachment and dysplasia. Some participants felt strongly that the chronic inflammation surrounding the lens should be included in the diagnosis, but the majority felt the inflammation was mild and relatively insignificant compared to the severe anatomic disorder present in this remarkable eye.
References:
- Coetzee P, Vuuren MV, Venter EH, Stokstad M. A review of experimental infections with bluetongue virus in the mammalian host. Virus Res. 2014; 182; 21-34.
- Gladden N, Rodriguez VG, Marchesi F, Orr J, Murdoch F. Multiple congenital ocular abnormalities including microphthalmia, microphakia and aphakia in a Simmental cross bull. Vet Record Case Reports. 2019; 7:e000702.
- Greene HJ, Leipold HW. Hereditary internal hydrocephalus and retinal dysplasia in Shorthorn Calves. Cornell Vet; 1974; 64; 367-375.
- Hocking PM, Guggenheim JA. The chick as an animal model of eye disease. Drug Discovery Today: Disease Models. 2013; 10(4); 225-230.
- Kaswan RL, Collins LG, Blue JL, Martin CL. Multiple hereditary ocular anomalies in a herd of cattle. J Am Vet Med Assoc. 1987; 191(1); 97-9.
- Leipold HW, Gelatt KN, Huston K. Multiple ocular anomalies and hydrocephalus in grade beef Shorthorn cattle. Am J Vet Res. 1971; 32; 1019-1026.
- Online Medelian Inheritance in Animals (OMIA). Accessed June 23, 2023. https://omia.org/home/.
- Siepker CL, Zimmer JL, Bedard KM, Hart KA, Czerwinski SL, Carmichael KP. Congenital cataracts and microphakia with retinal dysplasia and optic nerve hypoplasia in a calf. Case Rep Vet Med. 2021;2064103.
- Uchida K, Kunieda T, Abbasi AR, Ogawa H, Murakami T, Tateyama S. Congenital multiple ocular defects with falciform retinal folds among Japanese Black Cattle. Veterinary Pathology. 2006; 43(6); 1017-1021.
- Van der Lugt JJ, Prozesky L. The pathology of blindness in new-born calves caused by hypovitaminosis A. Onderstepoort J. Vet Res. 1989; 56; 99-109.
- Wilcock BP, Njaa BL. Special Senses. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th St. Louis, MO: Elsevier; 2016: 419-420, 469-470.
- Vielle A, Park YK, Secora C, Vergara MN. Organoids for the study of retinal development and developmental abnormalities. Front Cell Neurosci. 2021;15:1-7.
- Fuhrmann S, Zou CJ, Levine EM. Retinal pigmented epithelium development, plasiticity, and tissue homeostasis. Exp Eye Res. 2014;141-150.