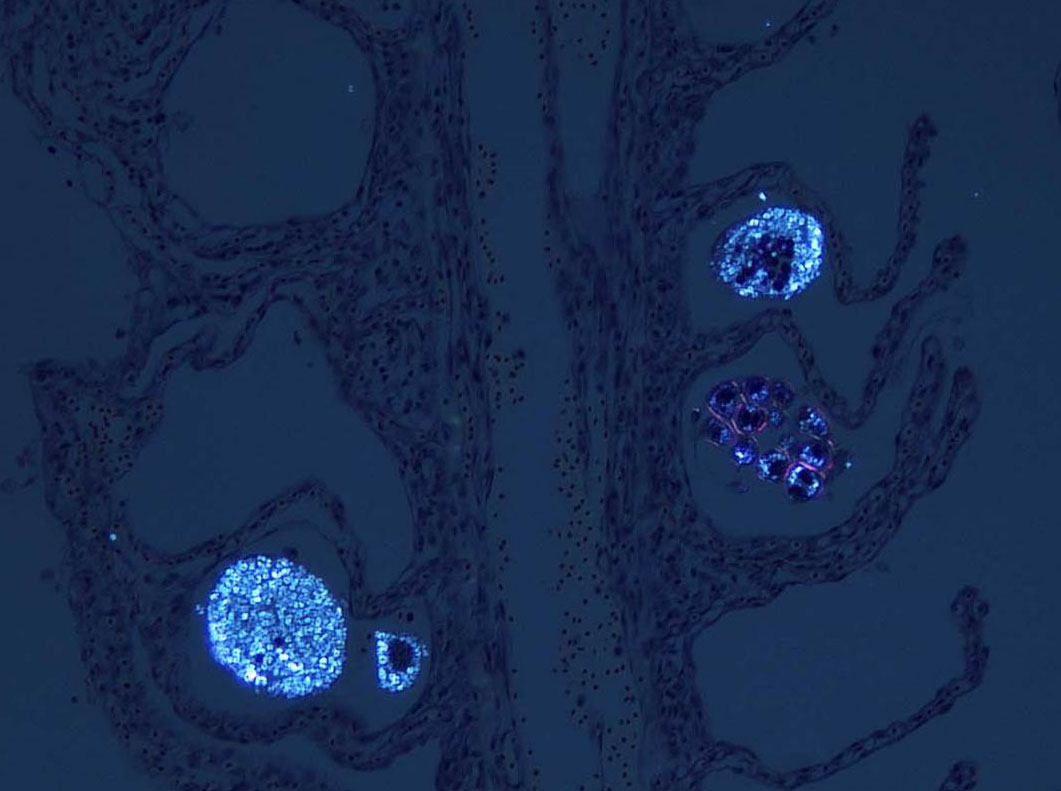
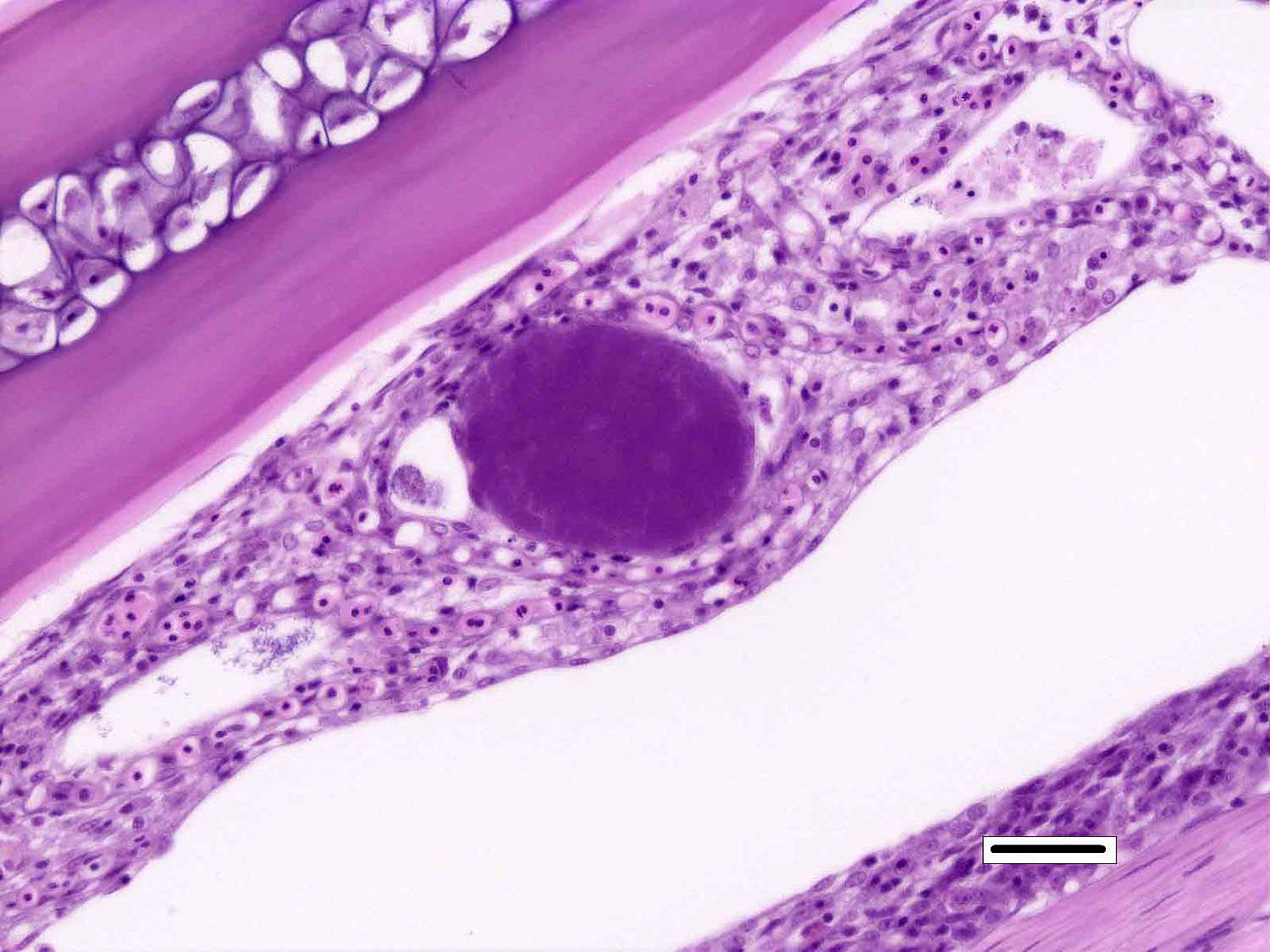
Signalment:
Gross Description:
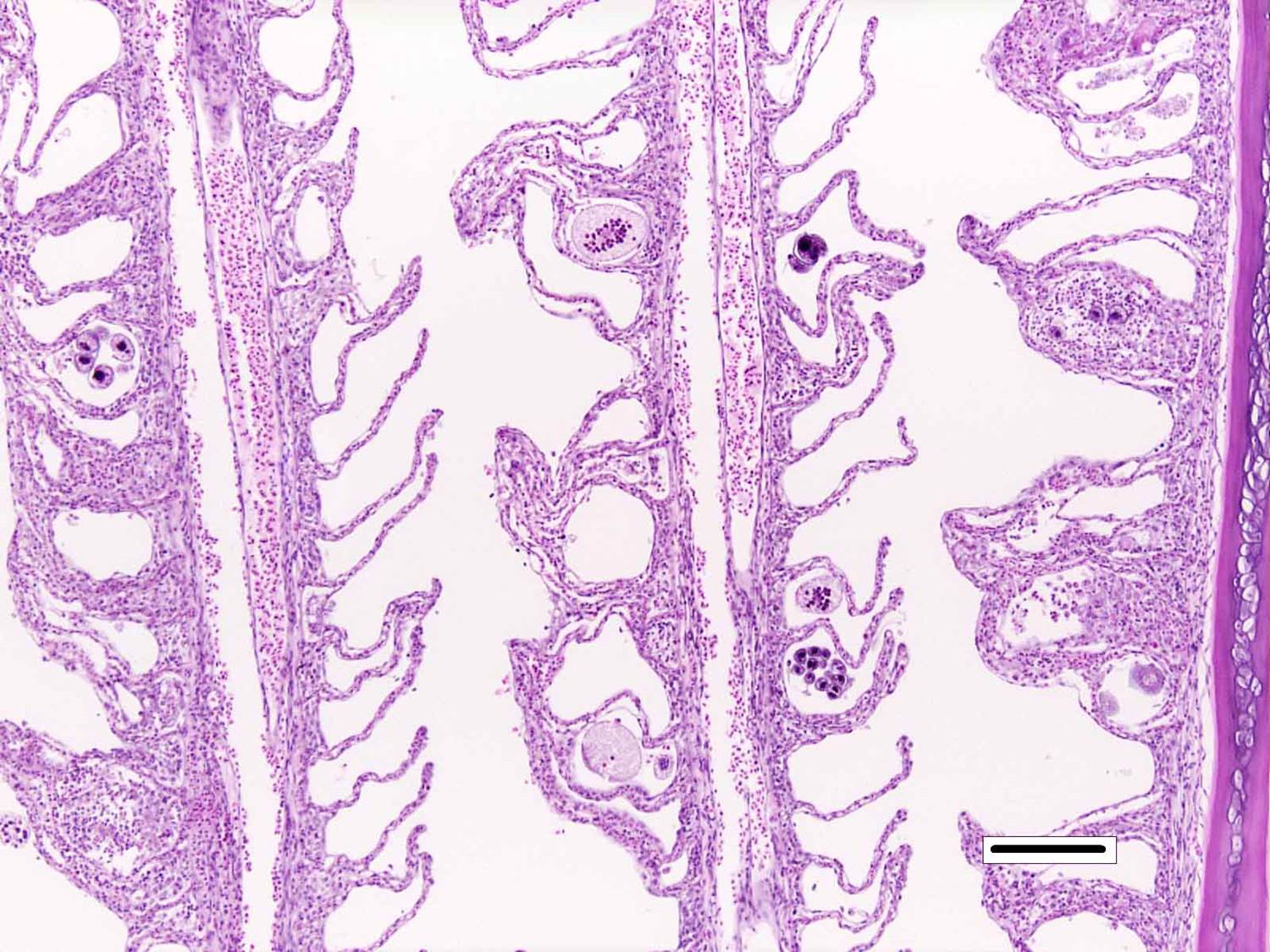
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
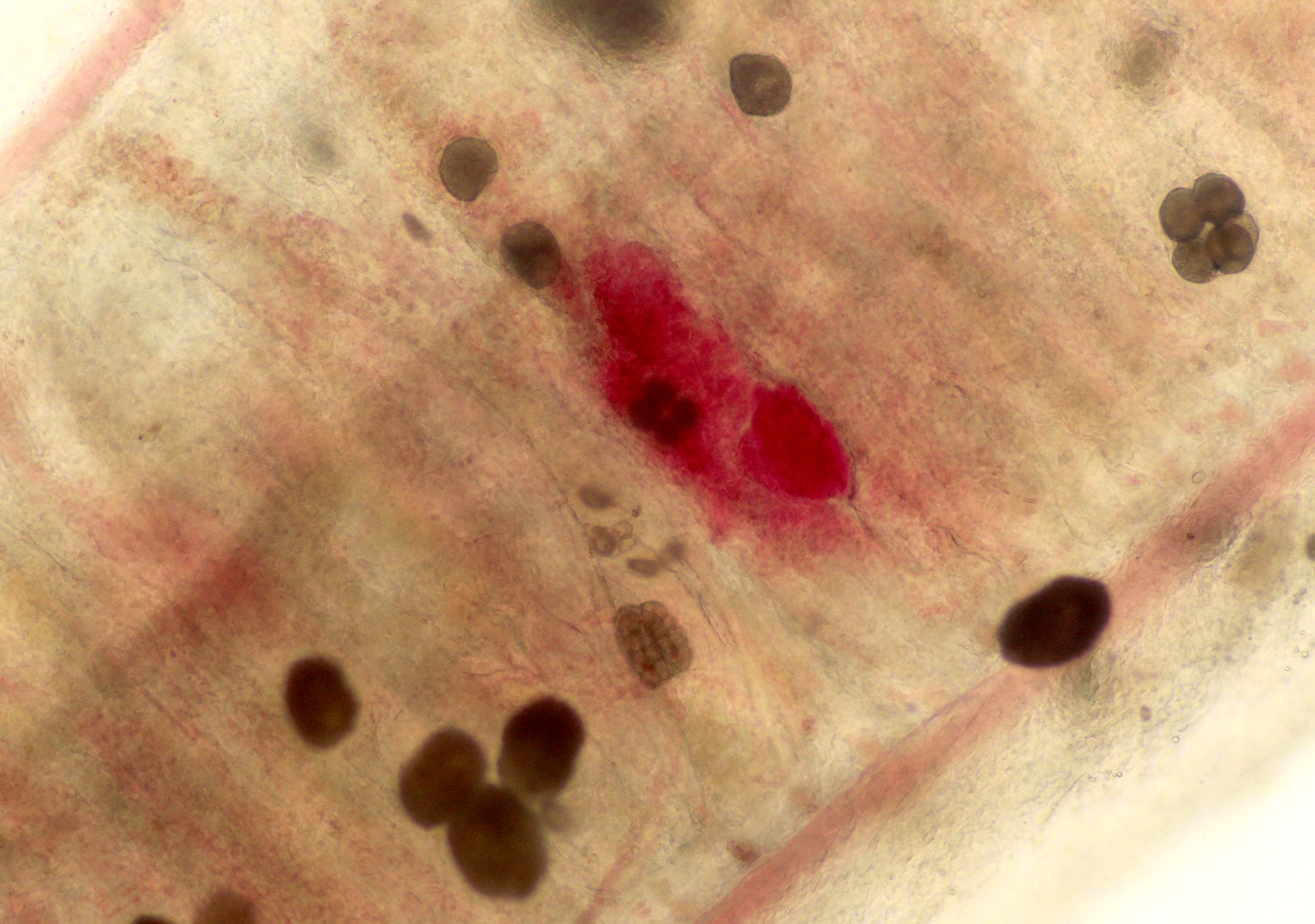
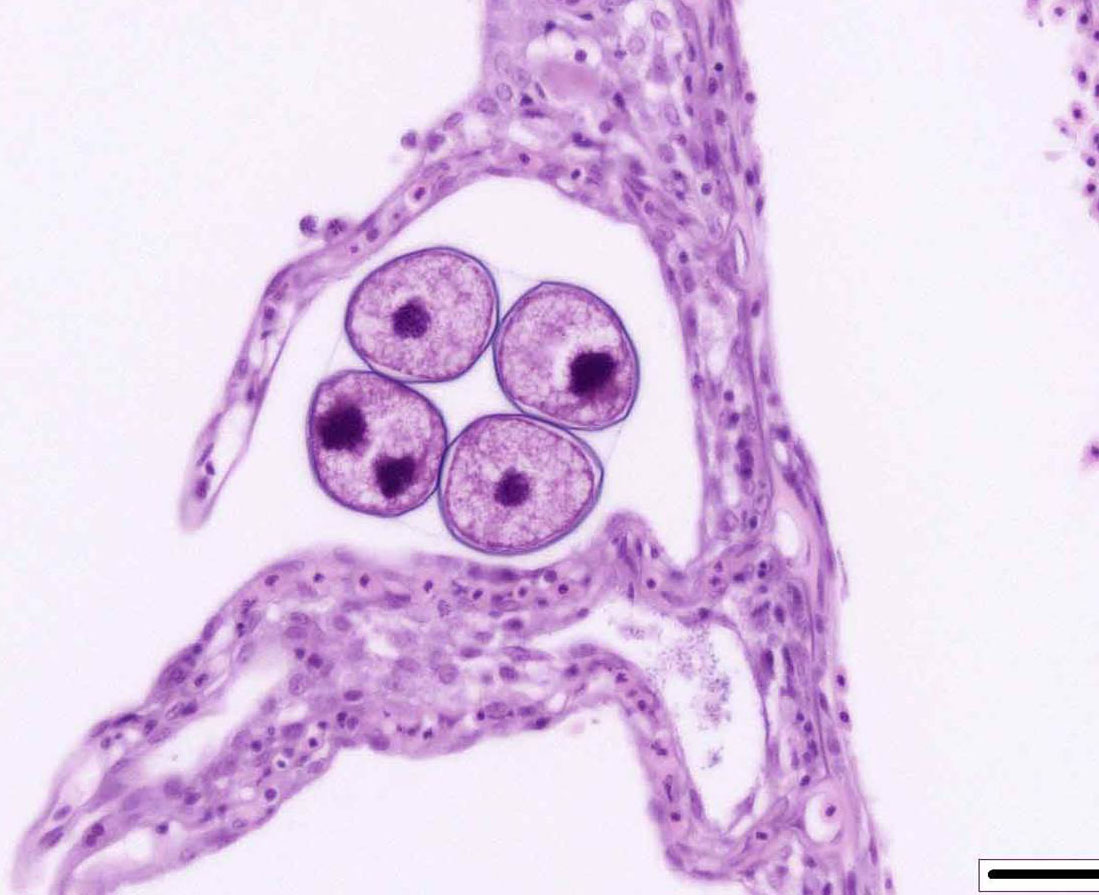
Contributor Comment:
The life cycle of A. ocellatum is direct and triphasic. The parasitic, feeding stage, or trophont, attaches to the host�s epithelium by a root-like structured called a rhizoid.5 After a period of feeding on the host, the trophont detaches from the host, retracts its rhizoid and becomes the encysted, dividing tomont stage in the substrate.5 The final stage is the free-swimming, infectious dinospore that is released from the dividing tomont (up to 256 dinospores can come from a single tomont) and has flagella to facilitate swimming to find a suitable host.5 Amyloodinium ocellatum causes severe physical damage to the host cells through attachment of the trophont to epithelium, with the gill typically being the primary site of infestation.5,6 In cases with heavy parasite burdens, the skin and eyes can also be infected, sometimes producing the dusty appearance of the skin that accounts for the name �velvet disease.�5 In some reports infestation of larval fish affected only the skin rather than the gill.6 The gill was the tissue most severely affected in this puffer fish, although a few of the organisms were seen on a wet mount of the fin; cutaneous changes were mild in histologic sections. Trophonts may also be seen in the pseudobranch, branchial cavity, nasal passages, and gastrointestinal tract (if swallowed).5
Microscopic lesions of severely affected gill include epithelial hyperplasia, often with lamellar fusion and distortion, mild inflammatory infiltrates, hemorrhage and epithelial degeneration and necrosis,3,5,6 as seen in this case. The feeding activity and the detachment of large numbers of organisms can damage epithelium, and potentially result in mortality as a consequence of hypoxia, osmoregulatory imbalance and secondary bacterial infections.5 There are several modalities of treatment available, most of which only target the dinospore stage, which can make eradication of parasites difficult.5
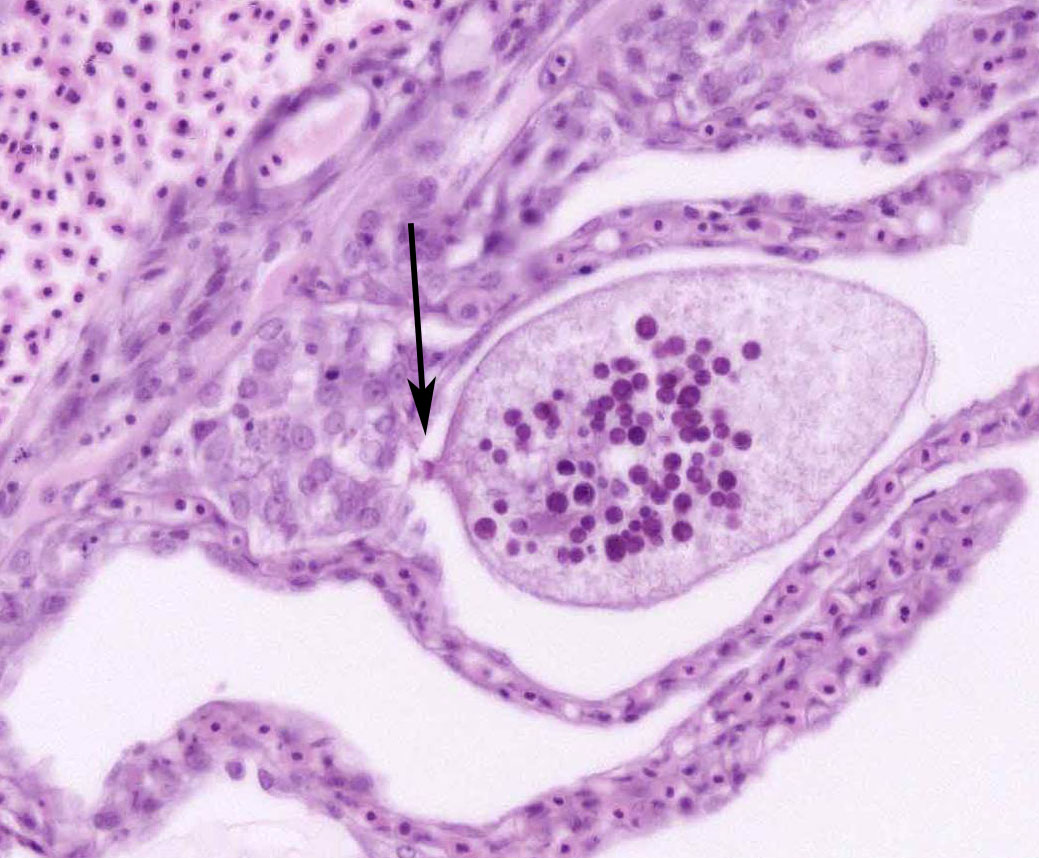
Although not considered the primary disease in this case, epitheliocystis was also noted in this fish. Characterized histologically by large, granular, basophilic, intracytoplasmic, bacterial inclusions within enlarged branchial lamellar epithelial cells, this type of infection is caused by a group of obligate, intracellular, gram-negative, chlamydia-like organisms.5 Recent advances in molecular techniques have identified various epitheliocystis agents as belonging to the order Chlamydiales, the first of which was Candidatus Piscichlamydia salmonis in the Atlantic salmon.1,7 Infections of gills, pseudobranch, and rarely the skin by these organisms have been described in many freshwater and marine species of fish.7 Pathogenicity is dependent upon the host response and the chlamydiales bacterium, and microscopic lesions can range from none to severe epithelial hyperplasia with associated inflammation.7
JPC Diagnosis:
2. Gill, lamellar epithelial cells: Chlamydial inclusions, multiple.
Conference Comment:
Prior to the conference, the moderator briefly reviewed the normal anatomy and histology of the gill. The gill arch is a series of bony or cartilaginous curved structures that support double rows of paired filaments, also called the primary lamellae. Each filament is composed of numerous perpendicularly-arranged secondary lamellae.4 The gill arch is covered by epidermis and at the origin of the primary lamellae the epidermis is thicker and contains many mucous cells and subjacent lymphoid tissue. The primary lamellae are covered by a mucoid epidermis which contains a pale-staining, eosinophilic, salt-secreting chloride cells that function in ionic transport and detoxification. The surface of the secondary lamellae consists of a single layer of interdigitating squamous epithelial cells supported by pillar cells and lamellar blood channels.4 The surface of the lamellar epithelium has numerous microvilli which serve as a substrate for cuticular mucus and aid in gas exchange and defense against infection and trauma. Thus, gills are important for gas exchange, ionic balance, and the excretion of the nitrogenous wastes. As a result, damage or infection of the gill often results in serious systemic con-sequences to the fish.4
Conference participants also discussed the difference between lamellar fusion and lamellar adhesion, both prominent features in this case. Lamellar fusion is most often associated with inflammation and is a consequence of marked epithelial proliferation. In contrast, lamellar adhesions (also known as lamellar clubbing or synechiae) occur when there is adherence of two or more secondary lamellae forming a pseudocyst, usually in the absence of epithelial proliferation or inflammation. Both lamellar fusion and lamellar adhesions are present in this case.
Multifocally, within the secondary lamellae of this case, there are dilated capillaries that contain organizing fibrin thrombi, in-terpreted by conference participants as examples of telangiectasia. The moderator noted that this vascular change can be a perimortem artifact secondary to capture of the fish or an antemortem change secondary to the disease process. Distinguishing between peri- and antemortem telangiectasis is often difficult, even for those experienced in gill histopathology; however, in this case, the presence of organizing fibrin thrombi is characteristic of antemortem telangiectasia rather than a perimortem capture artifact.�
References:
1.     Draghi A, Popov VL, Kahl MM, et al. Characterization of �Candidatus Pischichlamydia salmonis� (Order Chlamydiales), a Chlamydia-like bacterium associated with epitheliocystis in farmed Atlantic Salmon (Salmo salar). J Clin Microbiol. 2004; 42(11):5286-5297.
2.     Francis-Floyd R, Floyd MR. Amyloodinium ocellatum, an important parasite of cultured marine fish. SRAC. 2011; 4705:1-12.
3.     Kuperman, BI, Matey VE. Massive infestation by Amyloodinium ocellatum (Dinoflagellida) in a highly saline lake, Salton Sea, California, USA. Dis Aquat Org. 1999; 39:65-73.
4.     Mumford S, Heidel J, Smith C, Morrison J, MacConnell B, Blazer V. Fish Histology and Histopathology. US Fish and Wildlife Service, National Conservation Training Center. http://nctc.fws.gov/resources/course-resources/fish-histology/index.html. Accessed May 25, 2017.Â
5.     Noga EJ. Problem 27 Marine Velvet Disease (Amyloodiniosis, Marine Oodinium Disease, Oodiniosis). IN Fish Disease: Diagnosis and Treatment, 2nd ed, Ames, IA: Wiley-Blackwell; 2010:143-147.
6.     Paperna I, Steinitz AH. Amyloodinium ocellatum (Brown, 1931) (Dinoflagellida) infestations in cultured marine fish at Eilat, Red Sea: epizootiology and pathology. J Fish Dis. 1980; 3:363-372.
7.     Stride MC, Polkinghorne A, Nowak BF. Chlamydial infections of fish: Diverse pathogens and emerging causes of disease in aquaculture species. Vet Microbiol. 2014; 171:258�266.