WSC 2022-2023
Conference 21
Case 1
Signalment:
Adult, male, Belgian blue, Ox (Bos taurus)
History:
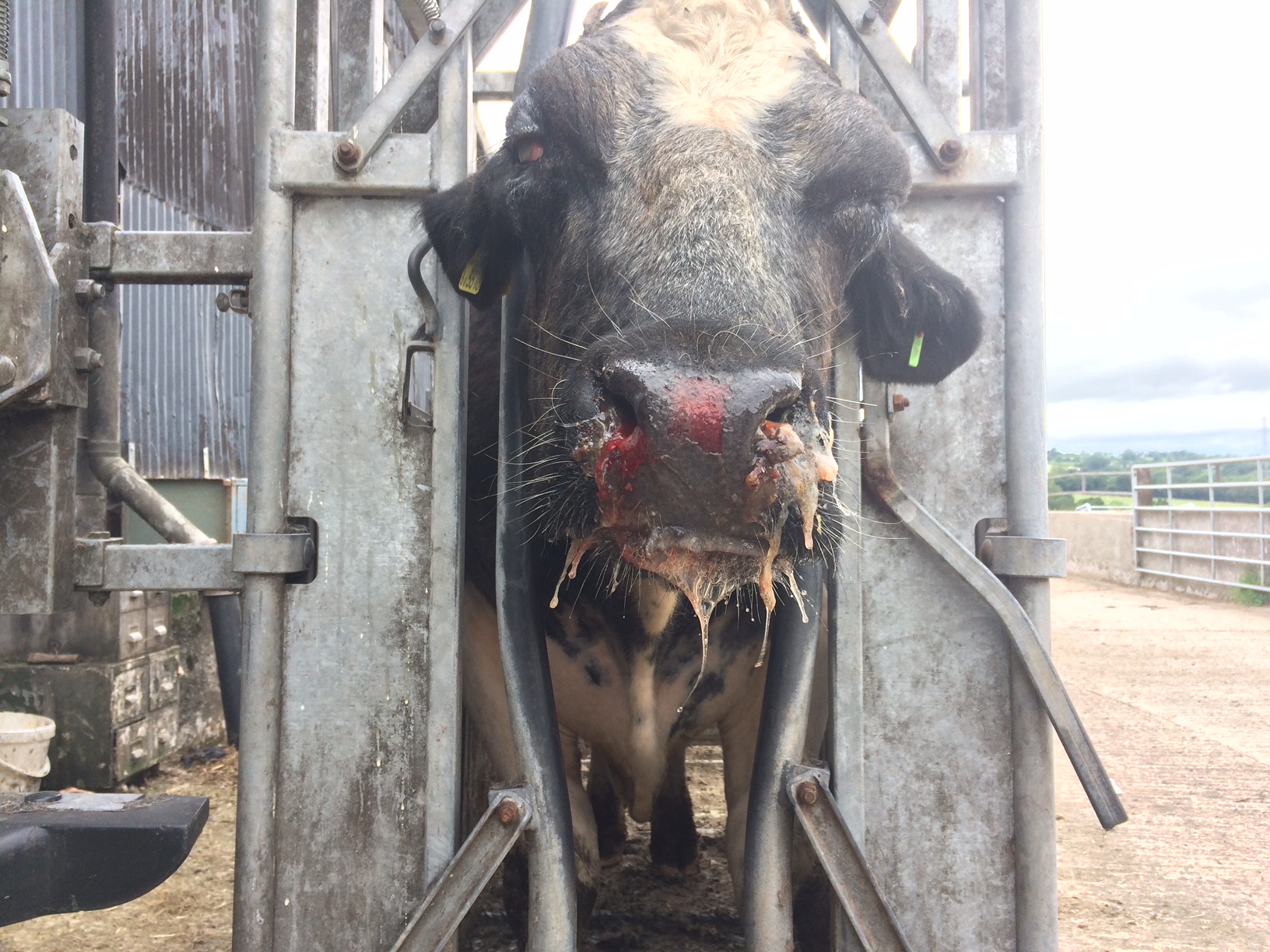
An adult Belgian blue bull was severely depressed, with severe bilateral keratoconjunctivitis, mucopurulent oculo-nasal discharge, and multifocal to coalescing erosive lesions on the muzzle. As the animal’s condition started to deteriorate rapidly, the decision to euthanize was taken by the owner.
Gross Pathology:
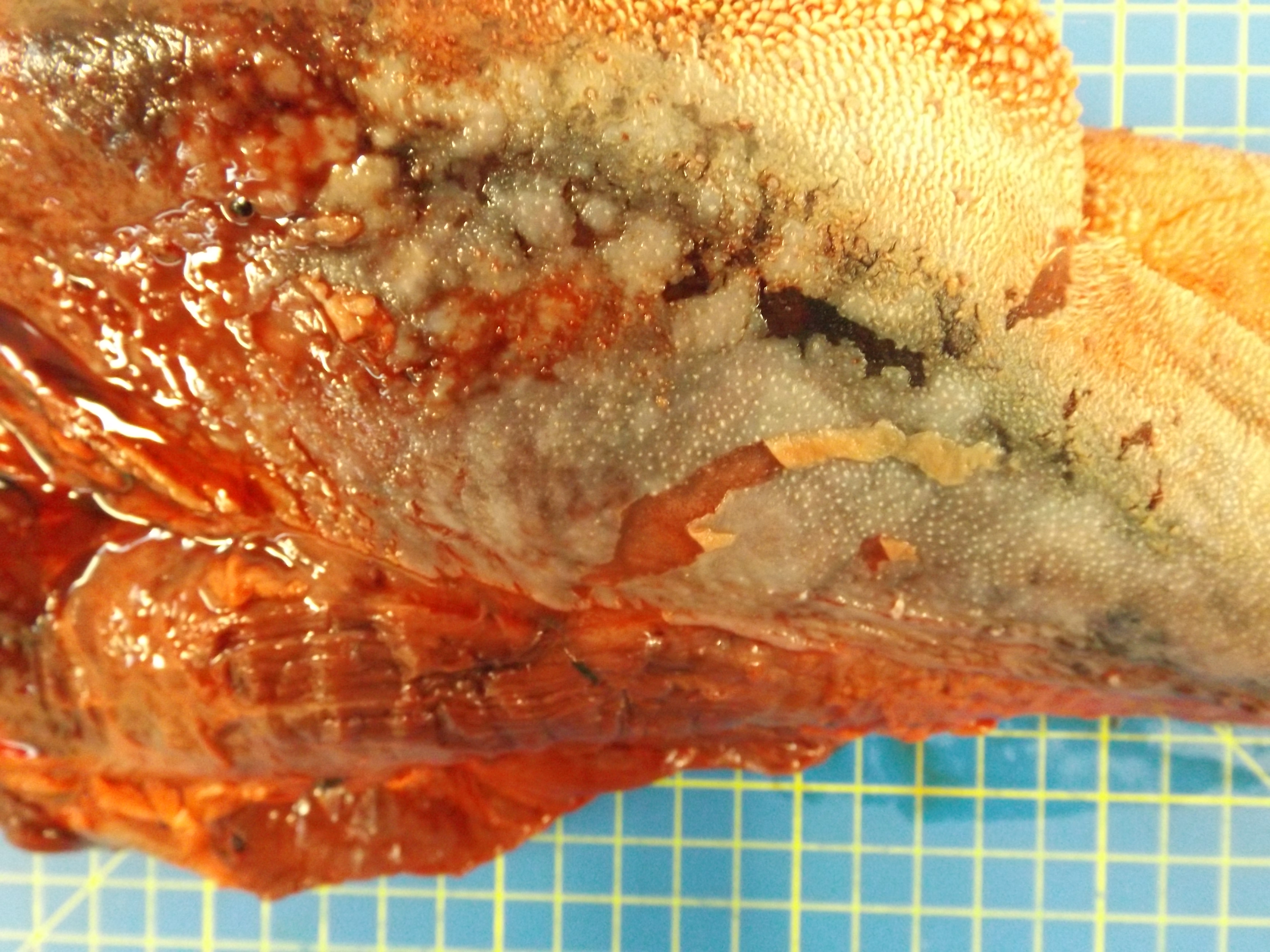
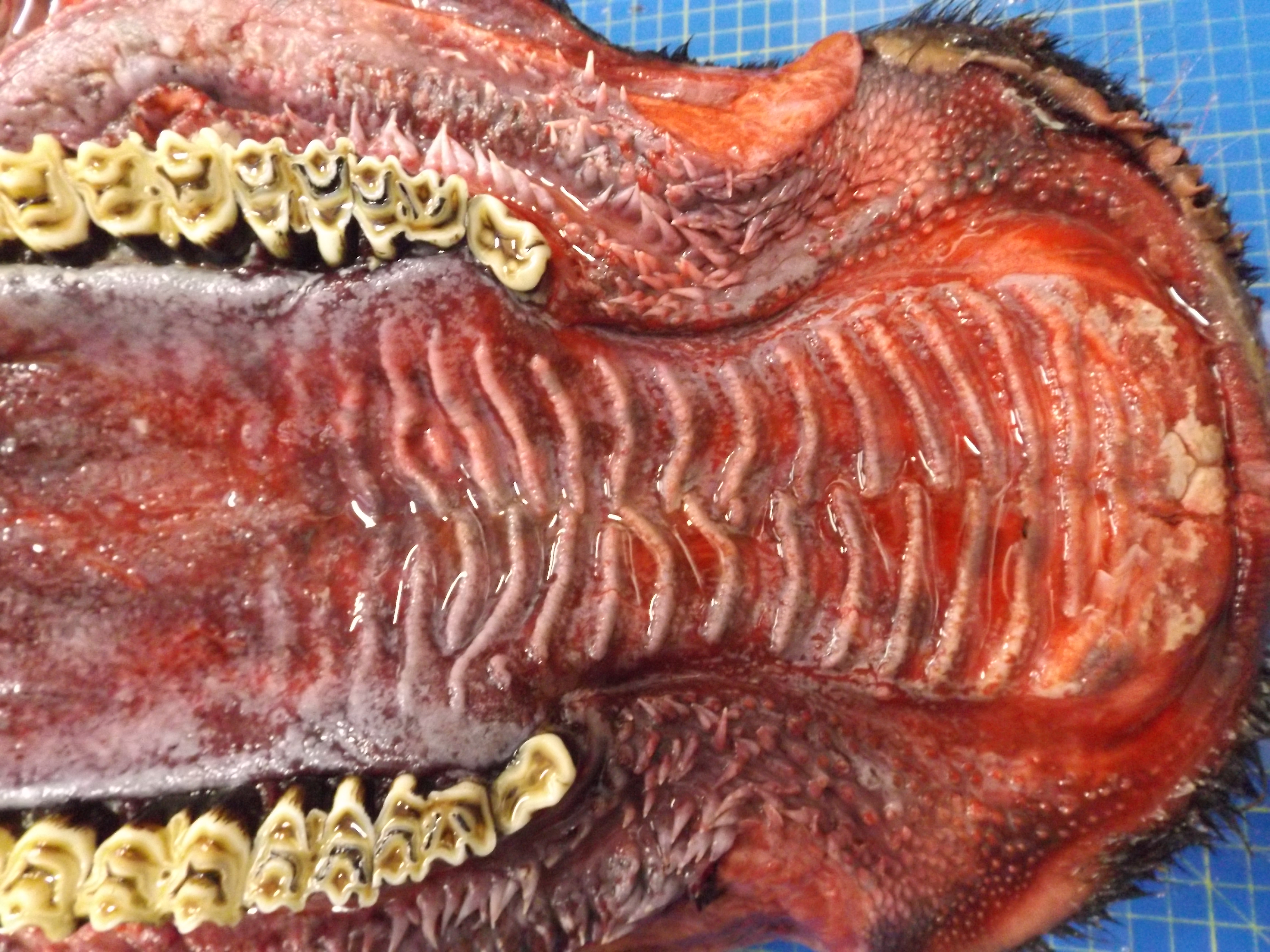
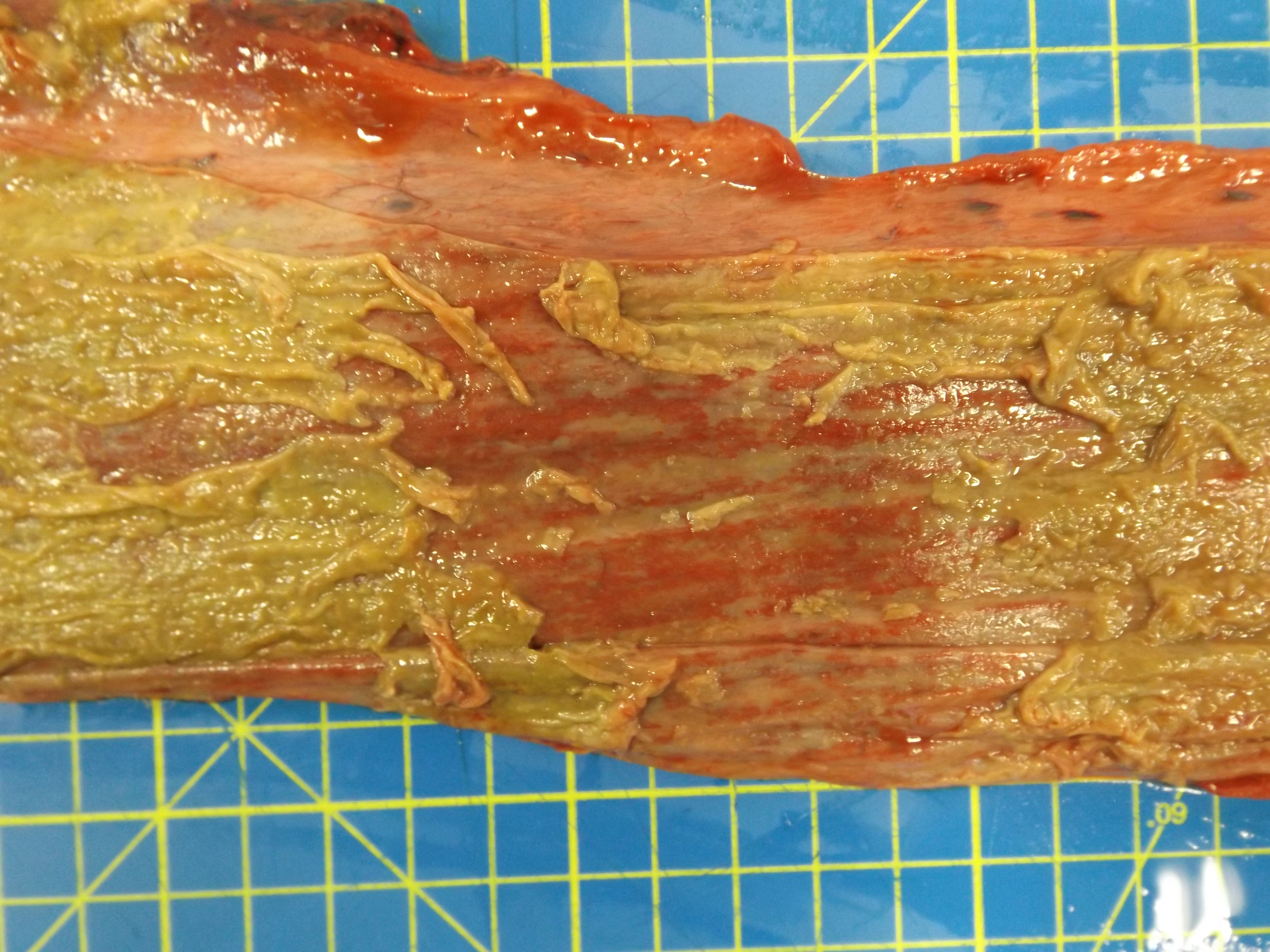
The animal was in poor body condition. There was bilateral conjunctivitis and multifocal, scattered, round, alopecic cutaneous lesions on the head. The oral mucosa exhibited multifocal, irregular to linear erosions, which were most pronounced on the tongue and the hard palate. The esophageal mucosa exhibited severe multifocal longitudinally oriented linear erosion and ulceration, with scattered multifocal hemorrhage. Generalized lymphadenomegaly and hyperemia of the vertebral rete mirabilis were also observed.
Laboratory Results:
PCR targeting the polymerase gene of AlHV-2 and OvHV-2 was carried out on samples from the tongue and lymph node. Both samples yielded a positive result for OvHV-2 DNA.
Microscopic Description:
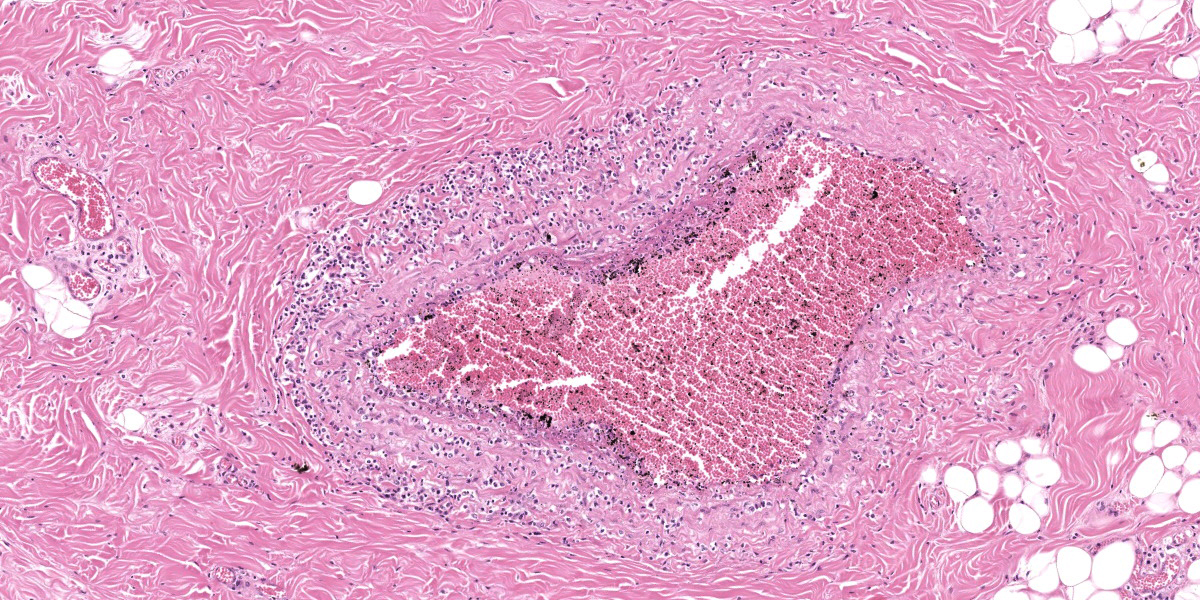
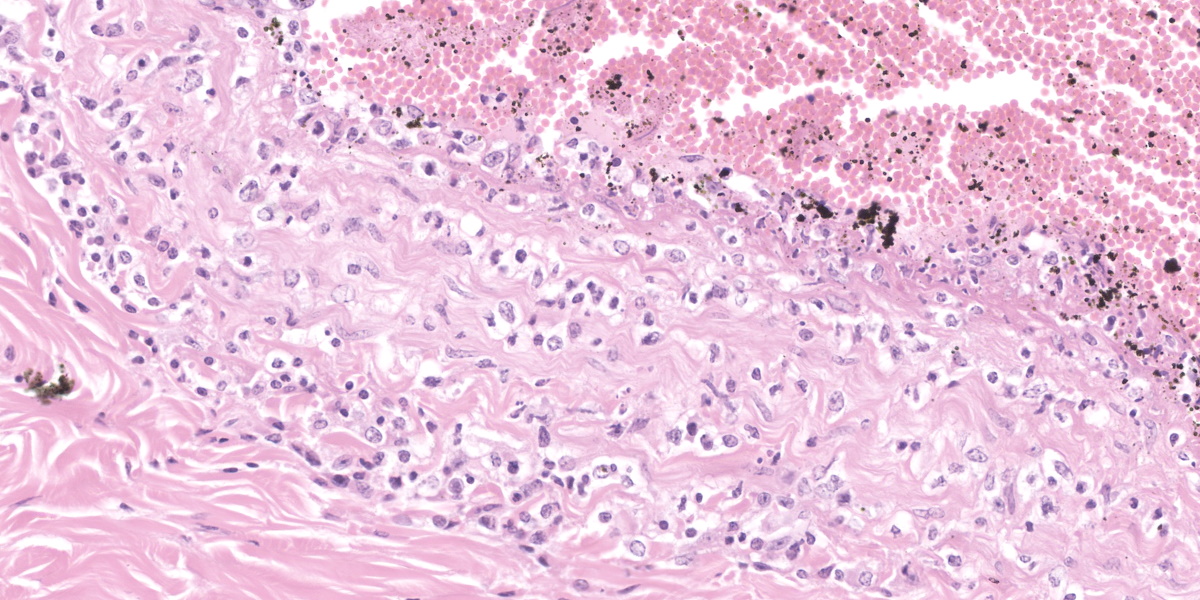
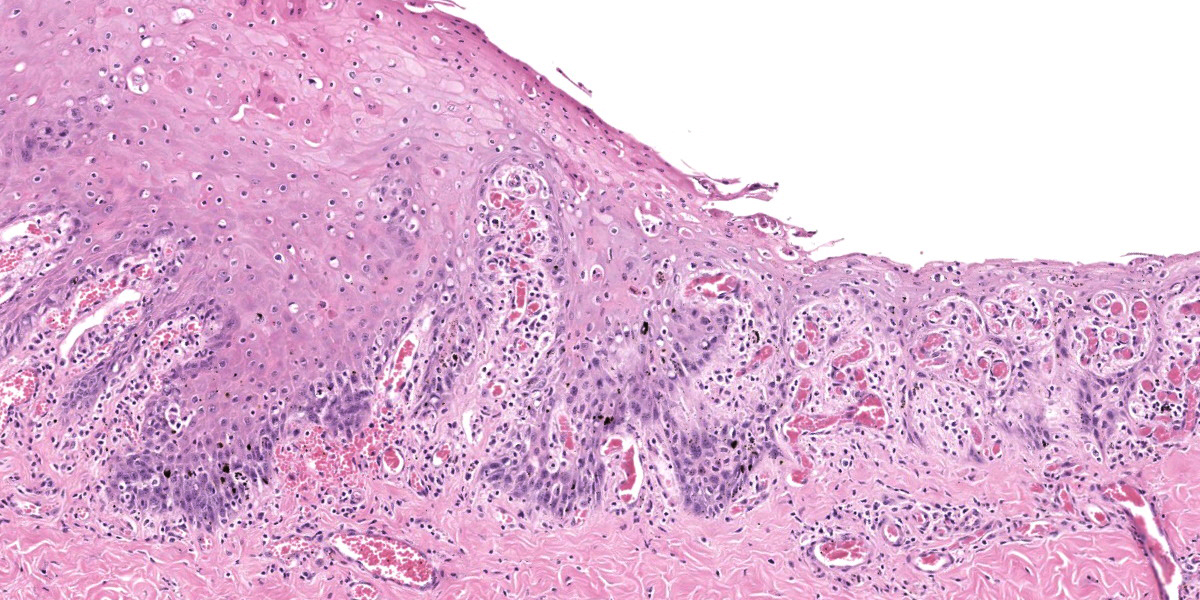
Oral mucosa. Diffusely, affecting both small and larger vessels (both arteries and veins), there is mild to moderate perivascular inflammation, composed of lymphocytes, plasma cells and fewer macrophages. The endothelial cells are often hypertrophic with plump nuclei which protrude in the vascular lumen. The tunica media of medium and large-sized arteries is expanded by moderate to large numbers of inflammatory cells and, in some arteries, there is multifocal accumulation of bright eosinophilic material (fibrinoid necrosis). The overlying oral mucosa shows focally extensive moderate intracellular and intercellular edema and necrosis, with erosion of the epithelial layer and sloughing of the epithelial cells and eosinophilic necrotic cellular debris. There is also mild to moderate multifocal lymphoplasmacytic infiltration of the epithelium. Inflammation and severe hyperemia are present in the superficial lamina propria, particularly extending between rete pegs.
Contributor’s Morphologic Diagnosis:
Oral mucosa: Moderate to severe diffuse subacute lymphoplasmacytic vasculitis with erosive stomatitis.
Contributor’s Comment:
Malignant catarrhal fever (MCF) is a viral disease which affects many different species of the order Artiodactyla, predominantly ruminants. Currently, several different viruses are considered causative agents of MCF; those which have been classified all belong to the genus Macavirus (malignant catarrhal virus), subfamily Gammaherpesvirinae and include Alcelaphine gammaherpesvirus 1 (AlHV-1), Alcelaphine gammaherpesvirus 2 (AlHV-2), Ovine gammaherpesvirus 2 (OvHV-2) and Caprine gammaherpesvirus 2 (CpHV-2) (https://talk.ictvonline.org/taxonomy/). These viruses, and others, including Ibex-MCF virus and another uncharacterized virus isolated from clinically affected white-tailed deer (WTD-MCFV) are responsible for natural outbreaks of MCF.4,8,13 Under experimental conditions, Hippotragine gammaherpesvirus 1 (HipHV-1) induced MCF in rabbits, although naturally occurring infections are not reported in this species.17 Gammaherpesviruses of ruminants are highly cell-associated lymphotropic herpesviruses. The key features shared among all the macaviruses, is the presence of the 15-A antigen epitope and high degree of similarity of the polymerase gene.13
The MCF-associated viruses have a recognized reservoir host, for example, sheep and wildebeest for OvHV-2 and AlHV-1, respectively. In the reservoir host, these viruses are usually clinically silent in contrast to susceptible species, in which infection causes clinical disease. The severity of the clinical signs varies greatly according to the species affected; some animals, for example bison (Bison spp.) or Pere’s David deer (Elaphurus davidianus), show a high susceptibility to disease, while others such as the white-tailed deer (Odocoileus virginianus), appear to be more resistant.11 The main source of infection is the reservoir host, in which infection is transmitted from adults to young animals at 2-3 months of age, while clinically susceptible species usually act as dead-end hosts; in the latter, however, vertical transmission and abortion has sporadically reported.5 Similar to other herpesvirus infections, the virus is shed through ocular and nasal discharges, which can then be inhaled or ingested via contaminated water or food. Even though the virus remains viable for only relatively brief periods outside the host animal, long distance transmission of OvHV-2 has been reported, causing MCF in bison.9
Clinical signs of MCF are similar, regardless of the causative gamma herpesvirus, but can be highly variable between individual animals. The most common and well-known expression of clinical disease is the “head and eye” form, in which the main clinical signs comprise depression, fever, keratoconjunctivitis, oral ulceration, lymphadenopathy and rhinitis with nasal discharge. However, gastro-intestinal, urinary, and neurological signs can be observed.18
Postmortem examination can reveal a wide range of lesions; bilateral keratoconjunctivitis, erosion of the nares with adhered crusts, and bilateral oculo-nasal discharge are typical gross findings. Cutaneous lesions consisting of exanthemata with overlying crusting are described on the thorax, abdomen, inguinal regions, perineum, and, less often, on the head.13 In the gastrointestinal system the most common lesions are observed in the oral cavity. Erosive lesions develop first on the lips, especially close to labial commissure, then develop on the tongue before extending over the oral mucosa, from the gingiva to the palate.13 Similar lesions can be observed also in the esophagus, the forestomachs and abomasum. The liver may show mild diffuse enlargement with multifocal pinpoint foci of white discoloration. Petechiae and erosions have also been described on the gall bladder mucosa. Respiratory lesions may be present, especially in the upper airway, ranging from mild congestion to fibrinous tracheobronchitis. Lesions in the urinary system are very characteristic and specific but are not always present; these consist of multifocal interstitial nephritis with infarcts giving a mottled appearance to the kidneys. In the lower urinary tract, erosion and hemorrhage are reported on the mucosa of the renal pelvis and the urinary bladder. Lymphadenomegaly is very common in most hosts and may be generalized or grossly affecting only a proportion of lymph nodes. Likewise, hemolymph nodes are affected and appear swollen. Edema of the meningeal vessels is also reported.18
The gross findings may be variable in distribution and severity, depending on the progression of the disease: acute MCF with rapid mortality is more likely to be associated with fewer grossly-evident lesions. In these cases, histopathology is an invaluable key diagnostic tool. Histologically, diffuse accumulation of mononuclear inflammatory cells around arteries and veins with fibrinonecrotizing vasculitis constitutes the hallmark of the disease; the distribution of these changes can be variable, affecting some tissues more severely than others, but are always present.13,18 The infiltrate is mainly composed of lymphoid cells with large, open nuclei and prominent nucleoli, with occasional plasma cells and small lymphocytes.18 Alongside these lesions, lymph nodes exhibit diffuse hyperplasia of the paracortical areas and necrosis and/or apoptosis can be observed in the skin, urinary and gastrointestinal tracts. Autolysis, accelerated by hyperthermia which occurs before death, can hamper diagnosis, especially in those cases where the lesions are mild.13
The gross and histopathological features are well recognized, however, the pathogenesis of MCF is not fully understood.7 Viral infection of susceptible hosts induces a lymphocytic proliferation, mainly targeting vessels, with the development of severe arteritis/phlebitis and consequent necrosis affecting several organs. The majority of these lymphocytes show a CD8+ immunophenotype, but only a small fraction show evidence of viral infection.13 Furthermore, large granular lymphocyte-like morphology and non-MHC restricted killing activity is observed in infected lymphocytes;1 in vitro experiments indicated that the cells are resistant to concanavalin A while responsive to cyclosporin A.7 Taken together, these findings seem to indicate that viral infection causes an autoimmune disease provoked by lymphocytic dysregulation, rather than a direct effect of the viral infection of lymphocytic cells. However, in experimental infection of bison an unexpectedly large number of virus-infected T cells was detected, suggesting that both direct viral effects and indirect immune responses may play a role in MCF pathogenesis.2,7,12 Further studies are needed in order to solve this discrepancy. Recently, the role of AlHV-1 sema, a member of the semaphorin protein family, has been studied. An immune-escaping activity has been shown in vitro; however, absence of viral AlHV-1 sema had did not impair MCF induction and associated lymphoproliferative lesions in experimentally infected rabbits.11
Contributing Institution:
Department of Veterinary Pathology and Public Health, Institute of Veterinary Science, University of Liverpool, Leahurst campus, CH64 7TE, UK
https://www.liverpool.ac.uk/vetpathology/
JPC Diagnosis:
Hard palate mucosa: Arteritis, lymphocytic and necrotizing, multifocal to coalescing, moderate, with ulceration.
JPC Comment:
This contributor provides a great description of this classic case of OvHV-2 malignant catarrhal fever in an ox. This week’s contributor, Dr. Patricia Pesavento of the University of California at Davis, discussed that when there is mucosal or epithelial ulceration without an evident cause on the surface, it is important to examine the arteries subjacent the ulcer in a search for vascular lesions. In this case, there is subtle vacuolation to the endothelium of the large artery within the section, and, more superficially, the medium caliber arteries are more severely affected with transmural, predominantly T-cells disrupting the arterial wall and lifting the intima, presumably arising from the capillary bed of the adventitia. The moderator also stressed the importance of being specific in vascular lesions: in this case, the lesion specifically affects medium caliber arteries, thus arteritis was included in the morphologic diagnosis as opposed to vasculitis. This localization is important in sorting other potential causes.
The moderator and conference participants discussed bovine viral diarrhea virus as a differential which would cause vascular lesions, and for that the gross images of the palate and esophagus, these would be reasonable differentials. The moderator also described how the herpesviruses which contribute to MCF are characteristically lymphoproliferative, so a pleomorphic population of lymphocytes may weigh more in favor of MCF. Aside from PCR for OvHV2 (which was conducted in this case and is the most common cause of MCF in the United States), IHC for BVDV or ISH for the MCF agent would allow for differentiation of these two entities.
There are some histologic features which point to a location more specific than oral mucosa: the distribution of submucosal adipose, the presence of an elastic artery close to the mucosal surface, and especially the undulating surface point to the hard palate.
While OvHV-2 typically does not cause clinical signs in the host-adapted species, in a 2018 Vet Pathol report, Dr. Pesavento et. al showed that OvHV-2 is associated with systemic necrotizing vasculitis and can sporadically cause arteritis in sheep similar to MCF in cattle.14 This study included a collection of cases previously diagnosed as idiopathic polyarteritis (“polyarteritis nodosa”) and using the in-situ hybridization probe specific for OvHv-2, demonstrated viral nucleic acid in lymphocytes associated with vasculitis in small to medium caliber arteries in all cases.14 This work showed that OvHv-2 driven MCF may occur in the adapted host.14
OvHV-2 associated MCF in cattle is generally fatal, and chronic infection and recovery are both considered rare.10,16 A histologic hallmark of chronic MCF in cattle is neointimal hyperplasia characterized by proliferation of spindle (myofibroblast) cells between the endothelium and internal elastic lamina; this may be accompanied by obliterative arteriopathy due to luminal narrowing.10,16 Other arterial lesions described in chronic MCF include disruption of the internal elastic lamina and attenuation or loss of the tunica media. A 2022 report in the Journal of Veterinary Diagnostic Investigation also reported lymphocytic hypophysitis in a chronic case of OvHV-2 associated MCF, and the lymphocytes positively labeled for OvHV-2 ISH.10
T-cells are described as the predominant inflammatory cell in the vascular lesions of
MCF; however, a recent study evaluating rete mirabile lesions of 34 cases of OvHV-2 MCF in cattle, water buffalo, and bison found that macrophages were present in equal or greater numbers to T cells in all cases.16 This raises the possibility that the pathogenesis of vascular damage and necrosis may be secondary to macrophage activity.16 This study also evaluated the expression of viral protein oLANA and ov-IL10 mRNA, products of latency-associated genes, and demonstrated that the virus infects many more cells than just T-cells: endothelial cells, smooth muscle cells, and macrophages all demonstrated immunohistiochemical staining for oLANA and in-situ hybridization for ov-IL10 mRNA.16
As the contributor describes, there are a group of MCF viruses from the genus Macavirus, sub-family Gammaherpesvirinae, family Herpesviridae, that cause MCF disease in susceptible hosts, and a recent report described an Ibex-MCF virus outbreak in captive duikers (small antelope).2 The virus was presumed to be spread indirectly from captive ibex in an enclosure 35 meters away, and cases in duikers were associated with times of parturition in the ibex.2 The disease was fatal, and clinical signs included anorexia, ataxia, and diarrhea or sudden death.2 On necropsy, mucosal ulceration was lacking, however, most of the animals had pulmonary ecchymoses, lymphadenomegaly, renomegaly, mucosal hemorrhage in the urinary bladder, and ascites.2 Half of the cases also had hydrothorax and hepatomegaly.2 Histologically, there were dense and wide perivascular lymphocytes and scattered (rare) fibrinoid vascular necrosis in multiple organs, demonstrating remarkable and chronic lymphoproliferation, with ibex-MCF identified in all cases using PCR and demonstrated in lymphocytes using ISH.2 Most of the animals were deficient in copper, and the authors raised the possibility of copper deficiency compromising the duikers’ immune responses, predisposing the animals to overwhelming viral infection.2 Clinically normal but infected ibex were culled and lacked any gross or histologic lesions associated with the virus.2
References:
- Burrells C, Reid HW. Phenotypic analysis of lymphoblastoid cell lines derived from cattle and deer affected with "sheep-associated" malignant catarrhal fever. Vet Immunol Immunopathol. 1991;29(1-2):151-161.
- Carvallo FR, Uzal FA, Moore JD, et al. Ibex-Associated Malignant Catarrhal Fever in Duikers (Cephalophus Spp). Vet Pathol. 2020; 57(4): 577-481.
- Cunha CW, Gailbreath KL, O’Toole D, et al. Ovine herpesvirus 2 infection in American bison: virus and host dynamics in the development of sheep-associated malignant catarrhal fever. Vet Microbiol. 2012;159(3-4):307-319.
- Gasper D, Barr B, Li H, et al. Ibex-associated malignant catarrhal fever-like disease in a group of bongo antelope (Tragelaphus eurycerus). Vet Pathol. 2012;49(3):492-497.
- Headley SA, Pimentel LA, Oliveira VH, et al. Transplacental Transmission of Ovine Herpesvirus 2 in Cattle with Sheep-associated Malignant Catarrhal Fever. J Comp Pathol. 2015;153(4):206-211.
- Hierwerger MM, Boujon CL, Kauer RV, et al. Cerebral Ovine Herpesvirus 2 Infection of Cattle is Associated with a Variable Neuropathological Phenotype. Vet Pathol. 2020; 58(2): 384-395.
- Li H, Cunha CW, Taus NS, Knowles DP. Malignant catarrhal fever: inching toward understanding. Annu Rev Anim Biosci. 2014;2:209-233.
- Li H, Dyer N, Keller J, Crawford TB. Newly recognized herpesvirus causing malignant catarrhal fever in white-tailed deer (Odocoileus virginianus). J Clin Microbiol. 2000;38(4):1313-1318.
- Li H, Karney G, O’Toole D, Crawford TB. Long distance spread of malignant catarrhal fever virus from feedlot lambs to ranch bison. Can Vet J. 2008;49(2):183-185.
- Milliron SM, Stranahan LW. Rivera-Velez AG, et al. Systemic proliferative arteriopathy and hypophysitis in a cow with chronic ovine herpesvirus 2-induced malignant catarrhal fever. J Vet Diagn Invest. 2022; 34(5): 905-908.
- Myster F, Palmeira L, Sorel O, et al. Viral semaphorin inhibits dendritic cell phagocytosis and migration but is not essential for gammaherpesvirus-induced lymphoproliferation in malignant catarrhal fever. J Virol. 2015;89(7):3630-3647.
- Nelson DD, Taus NS, Schneider DA, et al. Fibroblasts express OvHV-2 capsid protein in vasculitis lesions of American bison (Bison bison) with experimental sheep-associated malignant catarrhal fever. Vet Microbiol. 2013;166(3-4):486-492.
- O’Toole D, Li H. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet Pathol. 2014;51(2):437-452.
- Pesavento PA, Dange RB, Ferreras C. Systemic Necrotizing Vasculitis in Sheep is Associated with Ovine Herpesvirus 2. Vet Pathol. 2018; 56(1): 87-92.
- Russell GC, Stewart JP, Haig DM. Malignant catarrhal fever: a review. Vet J. 2009;179(3):324-335.
- Saura-Martinez H, Al-Saadi M, Stewart JP, Kipar A. Sheep-Associated Malignant Catarrhal Fever: Role of Latent Virus and Macrophages in Vasculitis. Vet Pathol. 58(2): 332-345.
- Schock A, Reid HW. Characterisation of the lymphoproliferation in rabbits experimentally affected with malignant catarrhal fever. Vet Microbiol. 1996;53(1-2):111-119.
- Uzal FA, Plattner BL, Hostetter J. Malignant catarrhal fever. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. St. Louis, Missouri: Elsevier; 2016:131-136.