Signalment:
Adult, female, spayed, domestic short-haired, cat,
Felis catusThe cat had initially presented with multiple cutaneous and subcutaneous nodular swellings on the paws. Over a 2 year period, these nodules slowly increased in size, coalesced and the overlying skin often ulcerated and developed crusts. Similar, large nodular, firm, coalescing, often ulcerated lesions also developed on the head, deforming the face and along the trunk. There was no response to several courses of antibiotic treatment. The cat was eventually euthanized
Gross Description:
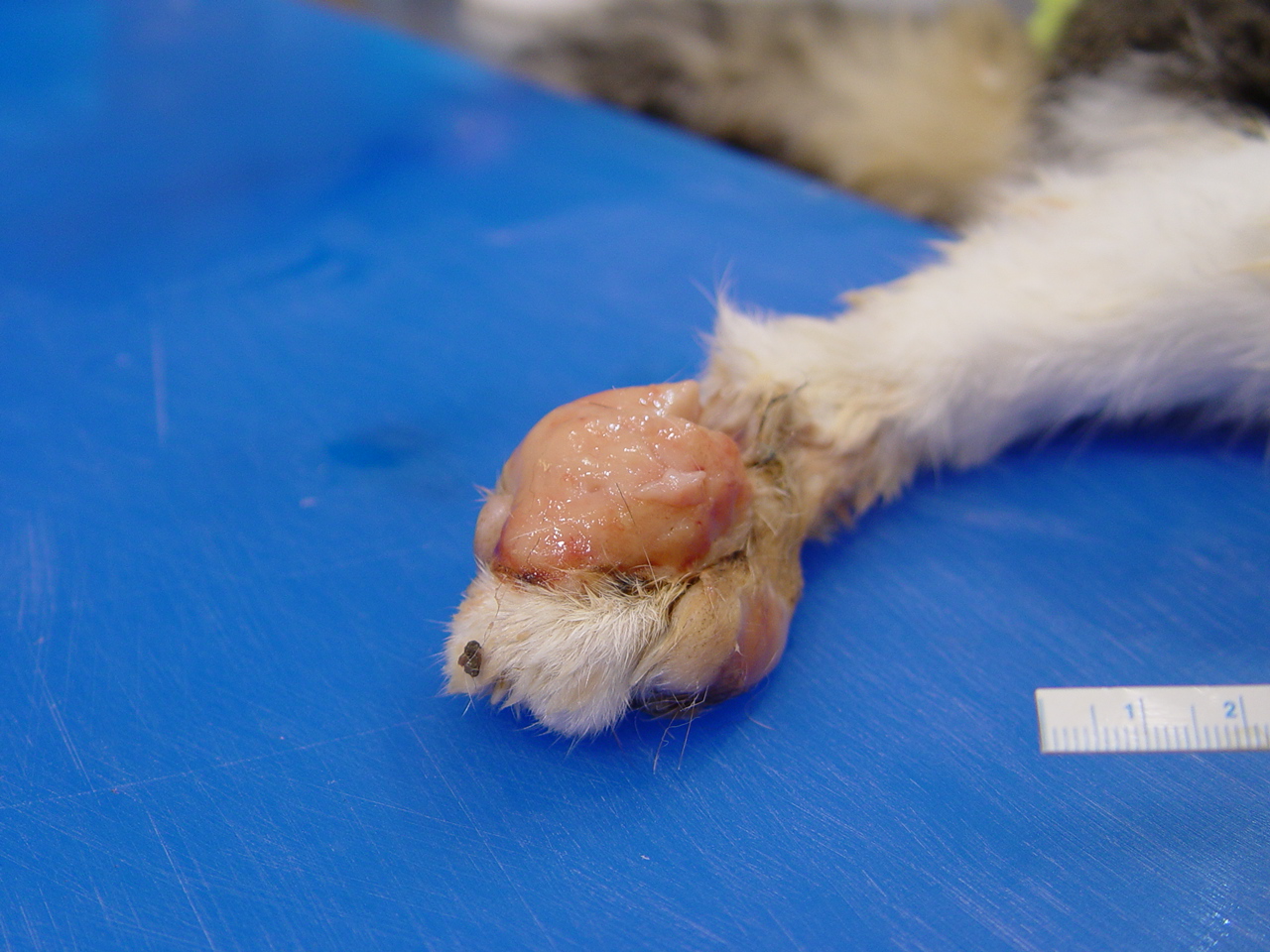
The cat was in good body condition having moderate visceral fat stores. Large, 4-5 cm in greatest diameter areas of the dorsal skin surface of both metatarsals were ulcerated and the skin was markedly thickened measuring up to 2.5-3 cm thick due to the presence of coalescing tan-colored nodules.. A similar, thickened tan colored, fleshy, ulcerated nodule measuring 3.5 cm in greatest diameter was present on the dorsal surface of the left front metacarpals
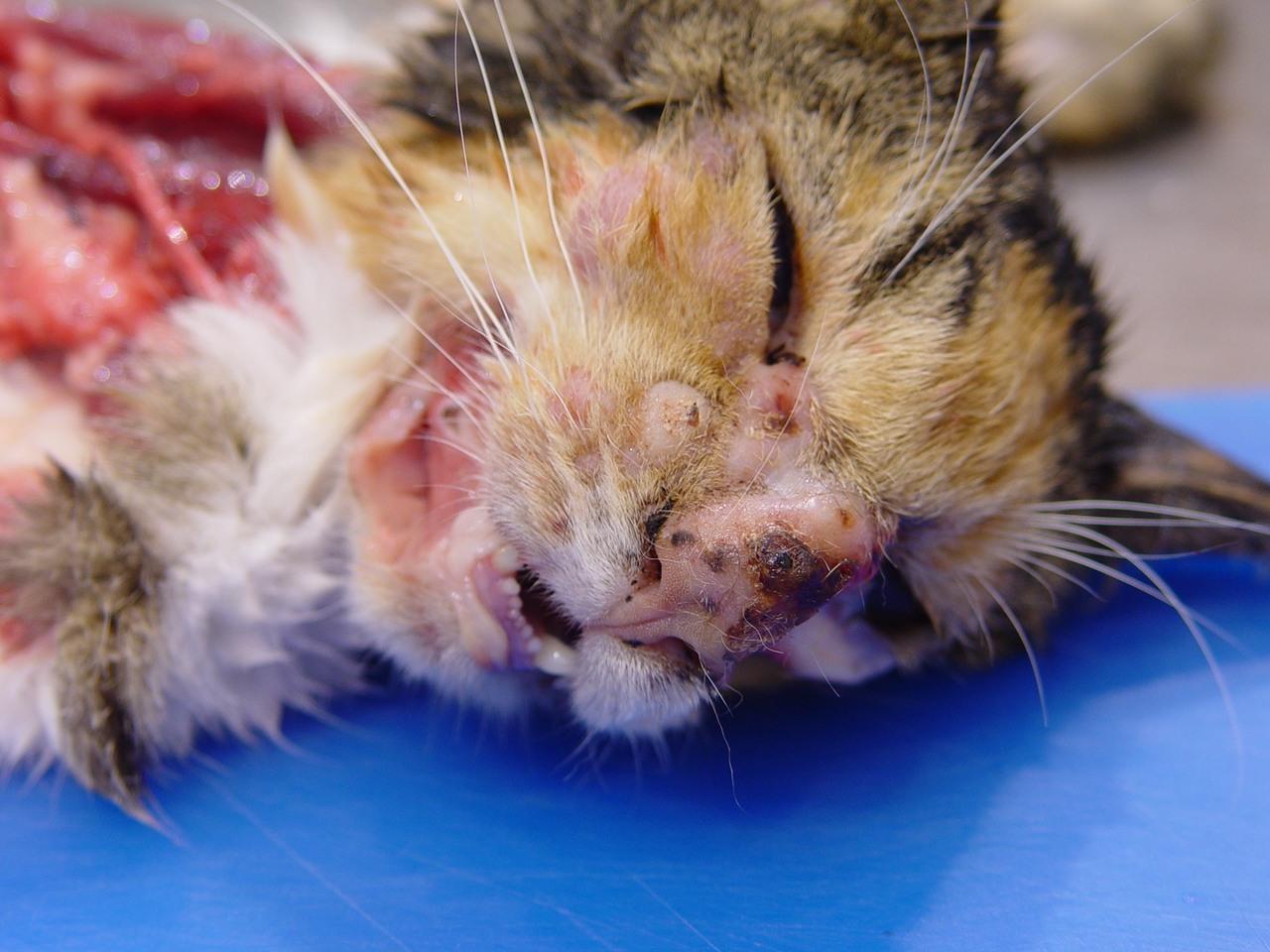
(Fig. 2-1). The skin extending medially and proximally to this area was alopecia and also mildly thickened. Multifocal to coalescing 5-10 mm in greatest diameter firm, nodules expanded the skin below the eyes. The bridge of the nose was ulcerated and the skin was markedly thickened, multinodular and firm
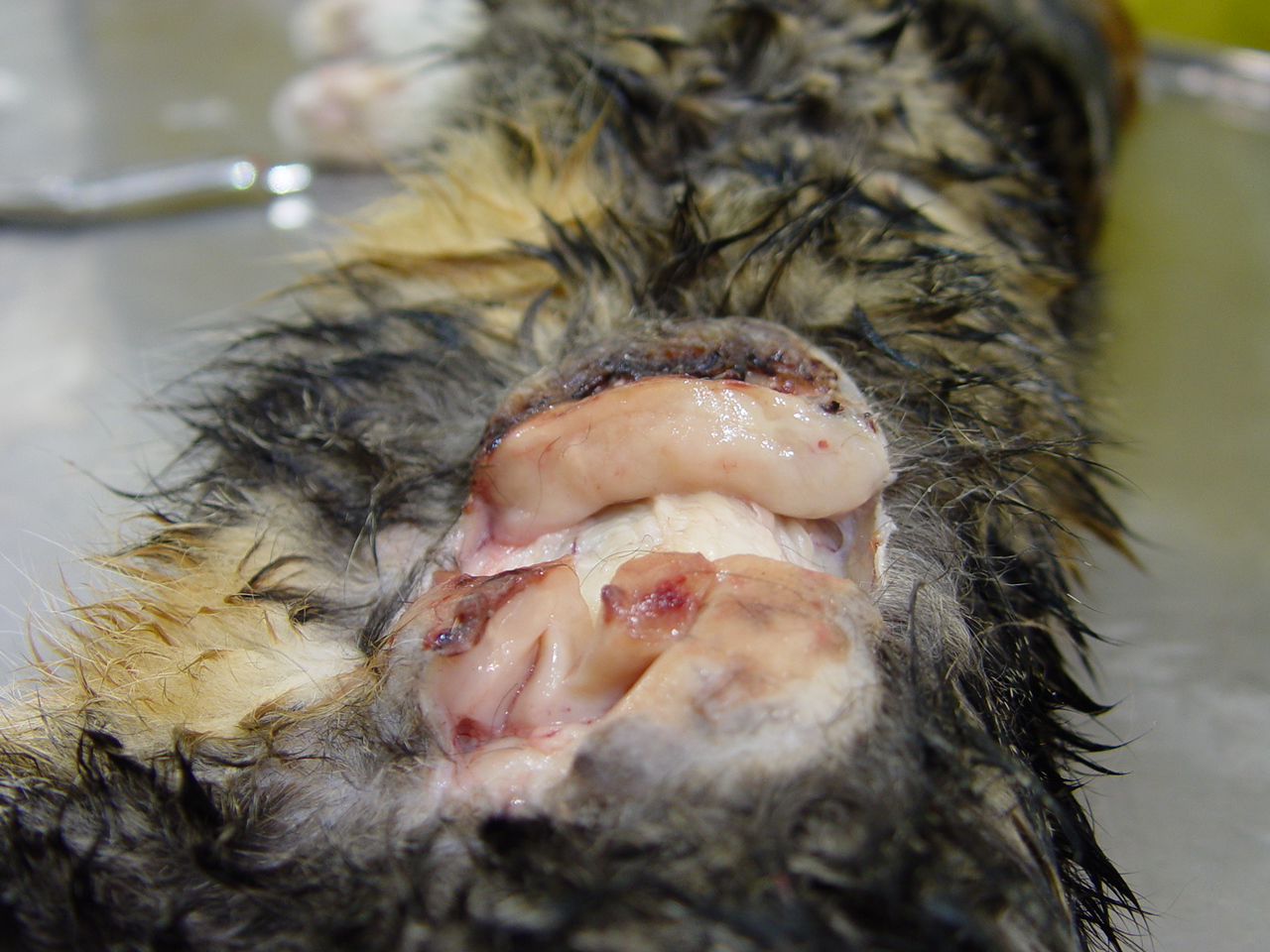
(Fig. 2-2). A large, 9 cm in greatest diameter and 2 cm thick, flattened, oval, ulcerated, nodule expanded the dermis and subcutis on the left flank
(Fig. 2-3). These lesions, with the exception of those on the bridge of the nose were freely moveable and on cut surface, were all pale, tan and had a homogenous texture. On the nose, this homogenous tissue extended into and partially effaced the underlying cartilage of the rostral, bridge of the nose and the nasal planum. In the area of the left popliteal lymph node, there was a pale, tan, 4-5 cm in greatest diameter tan nodule. The overlying skin was alopecic. There were several, freely movable, 3-4 cm in greatest diameter, subcutaneous, soft nodules in the mid to caudal ventral abdomen. The visceral organs were grossly unremarkable.Â
Histopathologic Description:
Sections of skin from lesions from the face, flank, left front and hind feet were examined. Sections of enlarged subcutaneous lymph node were included in some sections. Lesions were all similar in appearance and consisted of poorly-defined, infiltrative, densely cellular, monomorphic populations of large, polygonal to plump, slightly spindloid cells (resembling fibrohistiocytic populations) which had large, oval to sometimes slightly bean-shaped, nuclei with coarse chromatin, often a single, prominent nucleoli and moderate amounts of variably well-defined, eosinophilic cytoplasm. Anisokaryosis within these cell populations was mild and mitotic figures are occasionally seen (1-2 per 6 HPF). These cellular populations were interspersed with small numbers of lymphocytes, plasma cells, mast cells, occasional small lymphoid follicles and were supported by small amounts of fine, collagenous stroma. These infiltrates extended from the dermoepidermal junction to the panniculus markedly expanding and often effacing the tissue. The overlying epidermis was mildly to moderately acanthotic and often extensively ulcerated. The left popliteal lymph node was markedly enlarged and adherent to the overlying skin. The normal nodal architecture was largely replaced by similar, dense infiltrates of mononuclear cells in which these fibrohistiocytic cellular proliferations predominated. Small residual cortical lymphoid nodules remained, as did the outline of the capsule in areas. There were multifocal large areas of pale lytic necrosis within the node. Subcutaneous nodules from the caudal abdominal or inguinal area represented similarly affected and enlarged lymph nodes. Modified acid-fast and PAS staining of sections of skin and lymph node do not reveal infectious agents. Toluidine blue staining did not reveal cytoplasmic, metachromatic granules. Immunohistochemistry was performed. The majority of mononuclear cell infiltrates were CD18 positive, MHC II positive and negative for T and B cell markers. These findings would be typical of a histiocytic cell population. Numerous CD3 positive T lymphocytes and rare single and clusters of CD45 B cells were also scattered within these infiltrates.Â
Morphologic Diagnosis:
Severe, multifocal to locally extensive, cutaneous, atypical histiocytic proliferation with ulceration and regional lymph node infiltration, skin of distal limbs, face, and flank
Lab Results:
At postmortem, samples of skin and enlarged regional lymph nodes were sampled. Aerobic and anaerobic culture did not reveal significant pathogens.
Condition:
Feline progressive dendritic cell histiocytosis
Contributor Comment:
The large ulcerated, nodular skin lesions that were so grossly prominent were composed of densely cellular infiltrates of histiocytes or macrophages-like cells interspersed with fewer lymphocytes. Special stains did not reveal infectious agents (such as mycobacterium or fungi) and these infiltrates did not form classic granulomas but instead were arranged in dense sheets. Regional lymph nodes were also multifocally enlarged due to prominent infiltrates of these same histiocytic populations which largely respected lymph node architecture. The postmortem findings, immunohistochemical results and the clinical history in this case are highly suggestive of a rare condition recently reported in cats called Feline Progressive Dendritic Cell Histiocytosis (FPDCH)
1,2. Very few cases have been reported but the clinical features of those reported cats are very similar to those reported in this cat. Affected cats initially present with a solitary skin nodule, typically located on the head, neck or distal limbs. These lesions progress to multiple, non-painful nodules which may occur anywhere and which commonly become ulcerated. Nodules may wax and wane but complete spontaneous regression has not been reported. In general, nodules progress in size and may coalesce to form large plaques. Regional lymph node involvement is common in chronic cases. The cause of this rare condition has not been determined. Immune dysregulation and proliferation of cutaneous dendritic cells, such as in Canine reactive histiocytosis, is one possible pathogenesis. However, unlike the canine cases, rare reported attempts to treat affected cats with immunomodulatory drugs has been unrewarding. FPDCH may represent a low grade neoplastic proliferation of dendritic cells which slowly progresses over time.Â
JPC Diagnosis:
1. Haired skin and panniculus: Atypical histiocytic proliferation, diffuse, severe, with low to moderate numbers of lymphocytes, plasma cells and mast cells, domestic shorthair (Felis catus), feline.
2. Lymph node: Atypical histiocytic proliferation, severe (not included in all sections).
Conference Comment:
Feline progressive dendritic cell histiocytosis (FPDCH) resembles Langerhans cell histiocytosis in humans and is divided into two subgroups, epitheliotropic and nonepitheliotropic.
1 In epitheliotropic form, the cellular infiltrate extends from the dermis up to the basement membrane, with single or clusters of cells located within the epidermis. In nonepitheliotropic form, the infiltrate extends within the dermis up to but not beyond the basement membrane.
1 The exact lineage of proliferating dendritic cells is not known.
1,2
Histiocytic proliferative diseases may be reactive or neoplastic. Chronically, FPDCH clinically and morphologically resembles histiocytic sarcoma and may affect one or more internal organs.
1,2 This suggests that FPDCH should be considered an indolent, slowly progressive, cutaneous neoplasm that may disseminate to various organs.
2
The etiology of FPDCH is currently unknown but it is thought to be related to chronic antigen stimulation, although animals do not respond to immunomodulatory therapy.
2
References:
1. Feline progressive Dendritic Cell Histiocytosis.Â
In: Skin Diseases of the Dog and Cat, Clinical and Histopathologic Diagnosis. ed. Gross TL, Ihrke PJ, Walder EJ, Affolter VK, 2
nd ed., pp. 845-847. Blackwell Publishing Limited. 2005.
2. Affloter, VK, Moore PF: Feline Progressive Histiocytosis. Vet Pathol 43:646-655, 2006