Signalment:
1-year-old male rat snake (
Pantherophis sp.)Presented for inappetence and weight loss.
Gross Description:
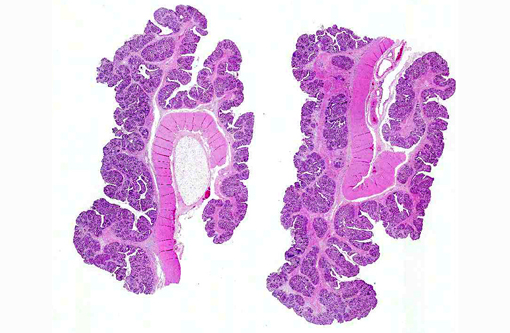
The mucosal surface of the stomach is diffusely tan and appears moderately thickened with a wall thickness of approximately 5 mm and increased prominence of rugal folds.
Histopathologic Description:
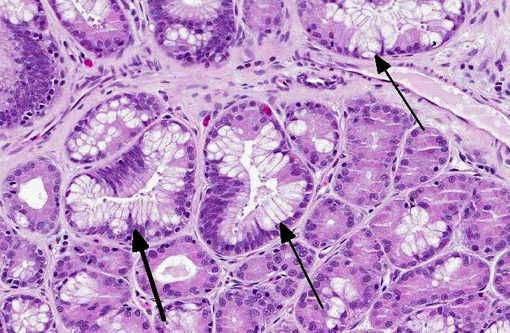
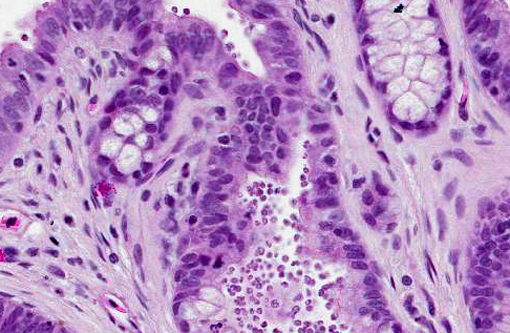
The gastric mucosa is diffusely moderately to markedly thickened, with prominent rugal folds. Gastric pits lined by luminal epithelial cells are moderately to markedly elongated and frequently mildly dilated and branched or irregularly shaped. There is moderate to marked proliferation (hyperplasia) of mucus neck cells and aggregates of mucus neck cells multifocally replace granular cells within deeper portions of gastric glands disrupting the normal glandular architecture. There is multifocal piling (hyperplasia) of luminal epithelial and mucus neck cells and low numbers of mitotic figures are observed within these cell populations (averaging one per 400X field) and these mitotic figures extend to the luminal aspect of gastric pits. Low to moderate numbers of gastric glands are mildly dilated. Low numbers of gastric glands lined by remaining granular cells have multifocal attenuation (atrophy) of the glandular epithelium. Large numbers of approximately 3-6 μm diameter protozoa with amphophilic cytoplasm and distinct basophilic nuclei are adherent to the luminal epithelium and epithelium lining gastric pits and glands and are frequently admixed with small amounts of mucin and low numbers of necrotic epithelial cells. The lamina propria is diffusely expanded by mildly to moderately increased amounts of fibrous connective tissue that separates adjacent glands. Low to moderate numbers of lymphocytes and plasma cells and low numbers of heterophils are scattered within the lamina propria and are present in multifocal, nodular aggregates that surround gastric glands. Low numbers of lymphocytes and plasma cells are present within the luminal and glandular epithelium. The submucosa is multifocally expanded by small to moderate amounts of pale amphophilic granular to wispy material (edema), mildly increased amounts of fibrous connective tissue and small numbers of similar inflammatory cells.
Morphologic Diagnosis:
Stomach: Hypertrophic gastritis, diffuse, moderate, chronic, with myriad intraluminal protozoa consistent with
Cryptosporidium sp.Â
Condition:
Cryptosporidium serpentes
Contributor Comment:
A number of
Cryptosporidium sp. infect reptiles, including
C. serpentis, C. varanii (saurophilum), and other unidentified species of
Cryptosporidium.(9) Despite this, each species of
Cryptosporidium is relatively species-specific; species from mammals do not infect snakes with experimental transmission.(6)
Cryptosporidium infection commonly causes proliferative gastritis, progressive weight loss, and eventual death.(4) In lizards, enteritis is more common, although gastritis can occur as well.Â
Histopathology is considered the gold standard for diagnosis, as it enables differentiation of pathogenic cryptosporidia from those ingested with prey, which makes fecal testing difficult. However, recent advances in molecular biology make fecal testing more useful.(9)
JPC Diagnosis:
Stomach: Gastritis, proliferative, diffuse, moderate, with marked mucus cell hyperplasia and metaplasia, rare mucosal epithelial necrosis and numerous intraepithelial and luminal apicomplexan schizonts.
Conference Comment:
Cryptosporidia are obligate intracellular coccidians of the phylum
Apicomplexa. Apicomplexans are so named because the sporozoites possess an apical complex, containing rhoptries, micronemes, a conoid and a polar ring associated with microtubules, all of which are evident ultra structurally.(2) These organisms reside within parasitophorous vacuoles along the epithelial microvillus border, and thus occupy an intracellular yet extracytoplasmic domain; at the junction with the host cell there are finger-like folds of parasitic cytoplasm formed within an electron dense attachment zone, known as the feeder organelle.(8) Infected hosts shed sporulated, thick-walled fecal oocyts that contain four sporozoites and can remain viable for several months. Upon ingestion, sporozoites excyst and invade the microvillus border of gastrointestinal, biliary, or respiratory epithelial cells, where they undergo asexual multiplication (schizogony/merogony) and gametogeny resulting in macro- and microgamonts. Finally, fertilization produces two types of oocysts, which sporulate within the host. Thick-walled oocysts exit the host, while thin-walled oocysts are involved in autoinfection; this ability to self-infect accounts for the chronicity and severity observed in some cases of cryptosporidiosis.(1)
Cryptosporidium infects both immune-competent and immune-suppressed animals.(3) Some cryptosporidia of veterinary importance are listed in table 1.(3,8,9)
Cryptosporidium spp. have a comparatively low minimum infective dose, are relatively resistant to normal concentrations of chlorination and can cause life-threatening diarrhea in immune-deficient humans.(5) Several species, most notoriously
C. parvum, are zoonotic; however the most frequent mode of human transmission is actually human-to-human.(7) Whereas dairy calves are an important potential source of
C. parvum, adult cattle typically shed non-zoonotic species.Â
C. meleagridis, especially from turkeys, has also been identified as zoonotic.Â
C. hominis and
C. parvum are the most commonly identified species in humans.(5) Interestingly,
Cryptosporidium sp. instigated the largest ever recorded waterborne disease outbreak, affecting 400,000 people (approximately one quarter of the population) in Milwaukee, Wisconsin in 1993.(7) Initially, contamination of the municipal drinking water was blamed on contaminated run-off from nearby cattle farms and abbatoirs; however
C. hominis was the primary isolate from water samples, with lower concentrations of
C. parvum, suggesting that the majority of the contamination was likely secondary to human sewage.(5)
Table 1. Selected
Cryptosporidium sp. (3,8,9)
| Species | Major Host |
| C. parvum | Mammals (human and bovine genotypes) |
| C. hominis | Humans |
| C. andersoni | Cattle, camels |
| C. suis | Pigs |
| C. felis | Cats |
| C. canis | Dogs |
| C. muris | Rodents, camels |
| C. wrairi | Guinea pigs |
| C. cuniculus | Rabbits |
| C. baileyi, C. meleagridis | Birds |
| C. serpentis, C. crotali | Snakes |
| C. varanii | Lizards, snakes |
| C. ducismarci | Tortoises |
| C. molnari | Fish |
| C. fayeri, C. macropodum | Kangaroos |
References:
1. Bowman DD, ed. Georgis Parasitology for Veterinarians. 9th ed. St. Louis, MO: Elsevier; 2009:99-101.
2. Brogden KA. Cytopathology of pathogenic prokaryotes. In: Cheville NF, ed. Ultrastructural Pathology: The Comparative Cellular Basis of Disease. 2nd ed. Ames, IA: Wiley-Blackwell; 2009:538-558.
3. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier; 2007:274-276.
4. Brownstein DG, Strandberg JD, Montali RJ, Bush M, Fortner J. Cryptosporidium in snakes with hypertrophic gastritis. Vet Pathol. 1977;14(6):606-617.
5. Chako CZ, Tyler JW, Schultz LG, Chiguma L, Beerntsen BT. Cryptosporidiosis in people: its not just about the cows. J Vet Intern Med. 2010;24(1):37-43.
6. Graczyk TK, Cranfield MR. Experimental transmission of Cryptosporidium oocyst isolates from mammals, birds and reptiles to captive snakes. Vet Res. 1998;29(2):187-195.
7. Leav BA, Mackay M, Ward HD. Cryptosporidium species: new insights and old challenges. CID. 2003;36(7):903-908.
8. Pohlenz J, Bemrick WJ, Moon HW, Cheville NF. Bovine Cryptosporidiosis: a transmission and scanning electron microscopic study of some stages in the life cycle and of the host-parasite relationship. Vet Pathol. 1978;15(3):417-427.
9. Richter B, Nedorost N, Maderner A, Weissenbock H. Detection of Cryptosporidium species in feces or gastric contents from snakes and lizards as determined by polymerase chain reaction analysis and partial sequencing of the 18S ribosomal RNA gene. J Vet Diagn Invest. 2011;23(3):430-435.
10. Richter B, Rasim R, Globokar Vrhovec M, Nedorost N, Pantchev N. Cryptosporidiosis outbreak in captive chelonians (Testudo hermanni) with identification of two Cryptosporidium genotypes. J Vet Diagn Invest. 2012;24(3):591-595.