Signalment:
Gross Description:
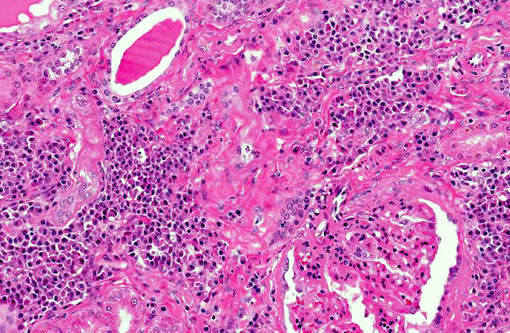
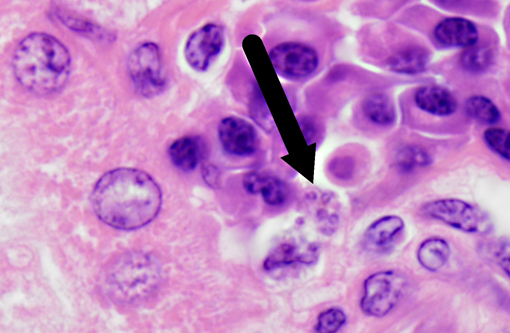
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Leishmania species have a unique pathogenesis and means of persistence within host cells enabling the establishment of long-term chronic infection. After a sandfly bite, an influx of both neutrophils and macrophages occurs, even in the absence of parasites. Parasites are able to survive within neutrophils due to the ability to inhibit the acidification of the phagosome, but have not been shown to transform into amastigotes or proliferate within in the neutrophil.(9) At the time of neutrophil apoptosis, surviving parasites are phagocytosed by resident and infiltrating macrophages, where the parasites will transform into amastigotes, replicate, and establish long-term infection. Dermal dendritic cells also become infected at the site of inoculation, becoming mature and migrating to the lymph node. Leishmania are resistant to acidification as amastigotes, and persist in these compartments which are late endosome associated LAMP1, Rab7 positive vacuoles.(13)
The immune response to all Leishmania species as an intracellular pathogen is dependent upon a timely and appropriate Th1 response including IL-12 production by dendritic cells and macrophages, efficient MHC II presentation, and subsequent IFNy production from T cell populations. Parasite killing is dependent primarily upon intracellular killing via superoxide and nitric oxide within phagolysosomes of infected macrophages. Leishmania utilize a number of immune evasion strategies to inhibit the immune response including the interruption of DC maturation, the stimulation of anti-inflammatory cytokines such as TGF-p and IL-10, the interruption of cellular signaling of the STAT pathways necessary for IFNy production, and through the induction of CD25+, FoxP3+ T regulatory cells.
Clinical presentation varies and includes dermal lesions, splenomegaly, generalized lymphadenopathy, cachexia, anorexia, muscle wasting, polyuria and polydipsia, proteinuria, keratoconjunctivitis, nail overgrowth, and hematologic abnormalities.(2,8,14) Splenic and hepatic lesions typically consist of granulomatous splenitis characterized by variable numbers of amastigote-infected macrophages, and lymphoplasmacytic and granulomatous portal and periportal hepatitis.(14) Skin lesions are one of the most common presenting signs in endemic regions and can include nonpruritic dermatitis, ulcerative dermatitis, focal or multifocal nodular dermatitis, proliferative dermatitis, or mucocutaneous ulcerative or proliferative dermatitis.(14) With these lesions, secondary bacterial pyoderma is the most common complicating co- morbidity.(8) Histologically, these lesions are granulomatous or pyogranulomatous with acanthosis, orthokeratotic and hyperkeratotic hyperkeratosis, and ulceration with serocellular crust formation.(8) Lymphoplasmacytic vasculitis and perivasculitis may also be present. Ocular lesions may also occur in approximately 16% of patients, depending on disease severity.(11) Common manifestations are conjunctivitis, blepharitis, and anterior uveitis.(11)
Renal disease due to glomerulonephritis and interstitial nephritis is a common clinical sign of canine leishmaniasis due to Leishmania infantum, occurring in greater than 96% of symptomatic dogs. Alterations in renal function during active VL are generally reversible with anti-Leishmania therapy with antimonials or amphotericin B.(1,7) However, VL-associated kidney disease is progressive and without therapy can result in end stage renal disease (>25% of canine cases).(7,12) Renal lesions due to visceral leishmaniasis have been previously characterized as progressive glomerulonephritis including mesangial proliferative, membranoproliferative (MPGN), focal segmental glomerulosclerosis, and minimal change glomerulonephritis, and a smaller percentage with crescentic glomerulonephritis.(4,5) Multiple studies have evaluated the morphology and ultrastructural characteristics of renal lesions due to canine leishmaniasis.(4,5) Previous characterizations described a wide array of morphologic changes, primarily of a membranoproliferative and mesangial proliferative type.(4,5)
Dogs with symptomatic VL typically have a hypergammaglobulinemia and a high degree of circulating parasite antigen.(3) It is logical that immune complexes comprise glomerular deposits responsible for VL-associated MPGN. However, studies evaluating the proteins associated with these glomerular deposits have had conflicting results, with either presence or absence of lgG, lgM, or C3b in glomerular deposits.(5,10) All studies found a significant increase in the amount of L. infantum antigen and inflammatory cells within the glomerular basement membrane and mesangium.(4,5) Increased numbers of CD4+ T cells within the glomerulus of affected animals as well as increased expression of adhesion molecules ICAM-1 and P-Selectin have been characterized.(5)
JPC Diagnosis:
Conference Comment:
While there are numerous potential clinical presentations of canine leishmaniasis, diffuse mesangioproliferative or membranoproliferative glomerulonephritis and interstitial nephritis are the most common renal manifestations.(1,4) As noted by the contributor, although a type III hypersensitivity mechanism has historically been accepted as the primary mechanism of VL glomerulopathy, there is new evidence to suggest that migration of CD4+ T-cells and increased expression of adhesion molecules such as ICAM-1 and P-Selectin are also involved, while decreased apoptosis may play a role in the proliferative pattern of MPGN.(5) The basic pathogenesis of type III hypersensitivity and immune mediated glomerulonephritis involves persistent antigenemia with a slight antigen excess, which results in circulating soluble immune complexes that deposit in glomerular capillaries. These antigen-antibody complexes activate the complement cascade via the classical pathway, which induces production of C3a, C5a and C5-9 (the membrane attack complex or MAC). C5a is chemotactic for neutrophils, which release toxic proteinases, arachidonic acid metabolites and oxygen free radicals, while both C3a and C5a are potent anaphylatoxins. Additionally, the MAC is capable of directly damaging the glomerular capillaries and mesangium.(16)
References:
2. Baneth G, Koutinas AF, Solano-Gallego L, et al. Canine leishmaniosis new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324-330.
3. Boggiatto PM, Ramer-Tait AE, Metz K, et al. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin Vaccine lmmunol. 2010;17:267-273.
4. Costa FA, Goto H, Saldanha LC, et al. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet Pathol. 2003;40:677-684.
5. Costa FA, Prianti MG, Silva TC, et al. T cells, adhesion molecules and modulation of apoptosis in visceral leishmaniasis glomerulonephritis. BMC Infect Dis. 2010;10:112.
6. Esch KJ, Petersen CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbial Rev. 2013;26:58-85.
7. lkeda-Garcia FA, Lopes RS, Ciarlini PC, et al. Evaluation of renal and hepatic functions in dogs naturally infected by visceral leishmaniasis submitted to treatment with meglumine antimoniate. Res Vet Sci. 2007;83:105-108.
8. Koutinas AF, Polizopoulou ZS, Saridomichelakis MN, et al. Clinical considerations on canine visceral leishmaniasis in Greece: a retrospective study of 158 cases (1989-1996). J Am Anim Hosp Assoc. 1999;35:376-383.
9. Laufs H, Muller K, Fleischer J, et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect lmmun. 2002;70:826-835.
10. Nieto CG, Navarrete I, Habela MA, et al. Pathological changes in kidneys of dogs with natural Leishmania infection. Vet Parasitol. 1992;45:33-47.
11. Pena MT, Naranjo C, Klauss G, et al. Histopathological features of ocular leishmaniosis in the dog. J Comp Pathol. 2008;138:32-39.
12. Plevraki K, Koutinas AF, Kaldrymidou H, et al. Effects of allopurinol treatment on the progression of chronic nephritis in Canine leishmaniosis (Leishmania infantum). J Vet Intern Med. 2006;20:228-233.
13. Rodriguez NE, Gaur Dixit U, Allen LA, et al. Stage-specific pathways of Leishmania infantum chagasi entry and phagosome maturation in macrophages. PLoS One. 6:e19000, 2011.
14. Solano-Gallego L, Koutinas A, Miro G, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165:1-18.
15. Weina PF, Neafie RC, Wortmann G, Polhemus M, Aronson NE. Old world leishmaniasis: an emerging infection among deployed US military and civilian workers. Clin Infect Dis. 2004;39(11):1674-1680.
16. Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:104-106,263-266,620-622.