Signalment:
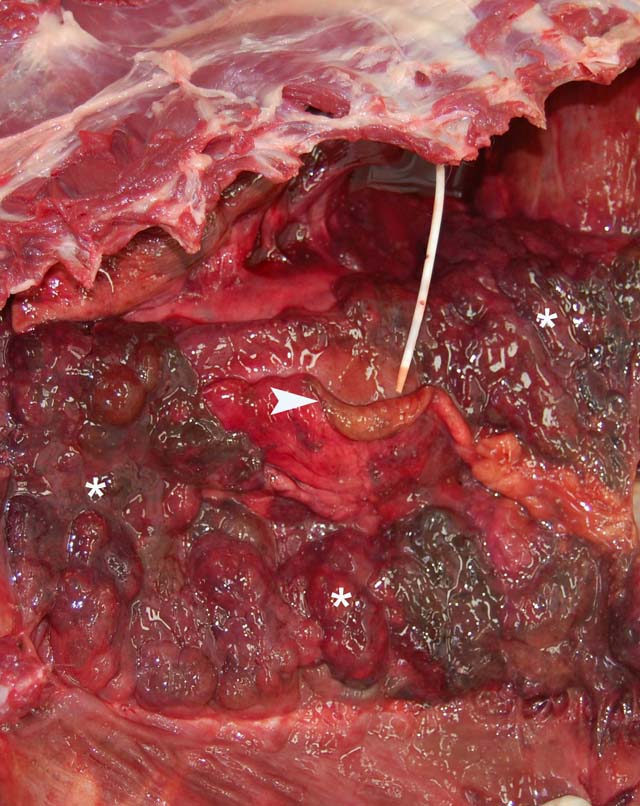
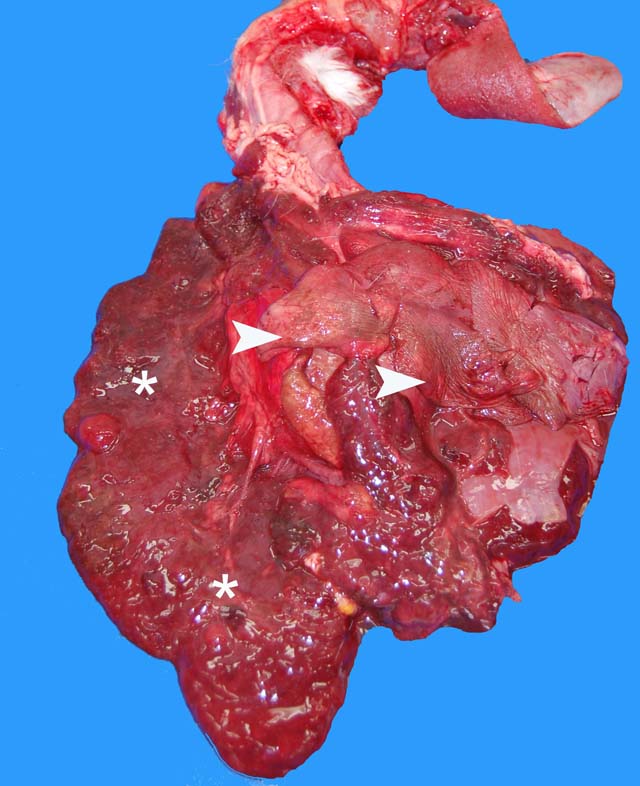
Gross Description:
Histopathologic Description:
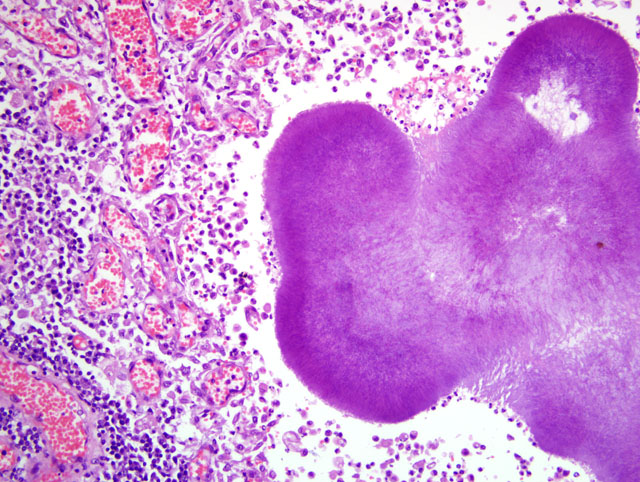
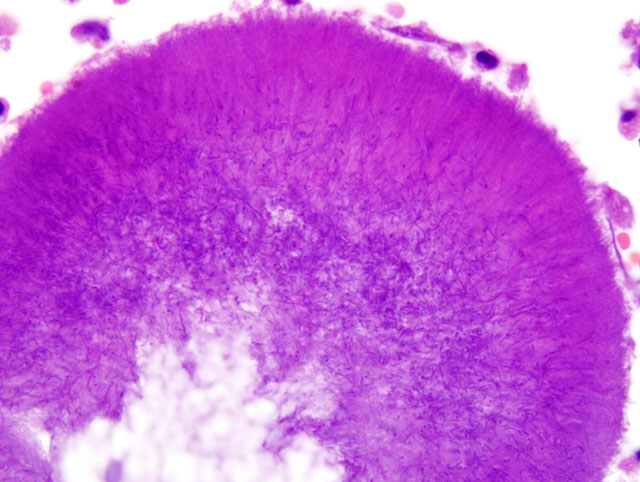
Parietal pleura: The pleura was diffusely expanded by infiltration with numerous histiocytes and neutrophils (pyogranulomatous inflammation), which surrounded intralesional large bacterial colonies composed of radiating filamentous bacteria. In addition, the pleura contained numerous small-sized congested vessels, mild fibroblast proliferation and multifocal infiltration with lymphocytes and plasma cells (Figs. 1-2; bacterial colonies are indicated by arrowheads).Â
Lungs: The visceral pleura was expanded by infiltration with numerous lymphocytes, plasma cells, epithelioid macrophages and scattered neutrophils, and contained numerous small-sized congested vessels and mild fibroblast proliferation. In the subpleural parenchyma, alveoli were lined by cuboidal cells (hyperplasia of type II pneumocytes). Scattered alveoli contained intraluminal macrophages and/or a few erythrocytes. Numerous alveolar spaces were decreased in size (atelectasis). There was mild anthracosis. Some pulmonary capillaries contained intraluminal megakaryocytes.Â
Morphologic Diagnosis:
1. Parietal pleura: Proliferative and pyogranulomatous pleuritis with intralesional large colonies of filamentous bacteria.Â
2. Lungs: Pyogranulomatous pleuritis and pulmonary atelectasis.
Lab Results:
Condition:
Contributor Comment:
Infection with either Actinomyces or Nocardia is associated with similar clinical signs and macroscopic and microscopic pathological findings.(10) Accurate ante mortem diagnosis of the agent involved by bacterial culture and/or molecular methods is important, since Actinomyces and Nocardia spp. require different antibiotic treatments.(3)
Actinomyces spp. are anaerobic or facultative anaerobic, Gram-positive filamentous bacteria, which are non-acid-fast.(3,7,13) They are commensal bacteria of the mucosa of the oral cavity, urogenital-, upper respiratory- and gastrointestinal tracts. Certain Actinomyces spp., e.g. A. hordeovulneris, are saprophytes, which are mostly attached to plant material such as grass awns.(3,7,10,12,13) Actinomycosis is caused by either endogenous or exogenous infection.(3,13) Endogenous infections are initiated by mucosal injury, through which Actinomyces spp. gain access to the underlying soft tissues. Exogenous infections result from contaminated bite wounds and inhaled or ingested grass awns with attached bacteria. The grass awns can migrate in tissues causing infections at distant sites.(3,7,13) Within infected tissues, Actinomyces usually spreads by direct extension; hematogenous dissemination is rare.(7) Bacterial spread is facilitated by proteolytic enzymes released from neutrophils and macrophages.(7) Actinomycosis in dogs usually causes (sub)cutaneous and intracavitary infections.Â
Subcutaneous lesions are often located in the cervico-facial or interdigital areas. Regional lymphadenopathy may be present. Intracavitary infections are mostly located in the thoracic cavity; infections of the abdominal cavity and the retroperitoneal space are uncommon.(4,7,10,12) In dogs, Actinomyces has also been reported as a rare cause of meningoencephalitis and endophthalmitis.(6,2) Actinomyces-induced pyogranulomatous meningoencephalitis has been reported in a 1-year-old German Shepherd dog without history of trauma or involvement of other organs by Actinomyces infection.(6) A migrating foxtail was considered as a possible cause for the meningoencephalitis.(6) Concurrent endophthalmitis and pneumonia due to Actinomyces infection have been described in a Rottweiler dog.(2) Actinomyces spp. were also isolated from canine corneas with ulcerative keratitis.(11)
Diseases caused by infection with Actinomyces spp. in other species include osteomyelitis of the mandible and maxilla in cattle (lumpy jaw; A. bovis and A. israelii), supra-atlantal (poll evil) and supraspinal (fistulous withers) bursitis of horses (A. viscosus and A. bovis together with Brucella suis or Brucella abortus), mastitis, abortion, pneumonia, cystitis and pyelonephritis in pigs (A. bovis, A. suis) and abscesses in brain and temporal bone in a goat.(1,3,8,13)
Actinomyces spp. are often isolated together with other commensal bacteria (7), particularly Bacteroides spp., Escherichia coli and Fusobacterium spp. The presence of additional bacteria results in an increased pathogenicity, since it facilitates the formation of bacterial aggregates, which are relatively resistant against phagocytosis and bactericidal enzymes released by inflammatory cells. Bacterial aggregates are formed by the attachment of fimbriae present on the surface of Actinomyces spp. to surface receptors of other bacteria.(7)
In comparison to Actinomyces spp., Nocardia spp. are aerobic and partially acid-fast Gram-positive bacteria, which exist in coccobacillary (resting phase) to filamentous (active growing phase) forms.(3) Infection with Nocardia is always exogenous; Nocardia spp. are present as saprophytes in the environment, where they are attached to soil, dust and plant material. Infection can be acquired by ingestion, inhalation and wound contamination.(3,7,13) In cattle, mastitis is usually caused by infection through the teat canal.(13) The primary localized infection may be followed by hematogenous dissemination.(3,7,13) The most common agent for nocardiosis in dogs is N. asteroides.(7) Nocardia infection causes three main disease manifestations: (sub)cutaneous, thoracic, and disseminated.(13) The (sub)cutaneous form is usually associated with lymphadenopathy of regional lymph nodes.(10) The thoracic form is characterized by the pleuritis and/or pneumonia.(3,13) Disseminated nocardiosis develops often secondary to the pulmonary disease.(7) Nocardial infections in other species include mastitis and abortion in cattle; pneumonia, lymphadenitis and abortion in pigs; and wound infection, mastitis and pneumonia in sheep, cattle and horses.(3,13)
The pathogenicity of Nocardia spp. is dependent on bacterial factors (strain and growth phase) and host immunity. Certain strains of Nocardia are more virulent due to an ability to survive in phagocytic vacuoles of neutrophils and macrophages.(3,7) Disease caused by Nocardia spp. is often associated with immune suppression or heavy bacterial exposure.(3,7)
Since infections with Actinomyces and Nocardia spp. cause similar lesions, the distinction between actinomycosis and nocardiosis is not possible based on macroscopic and microscopic pathological findings.(3,7,10) Macroscopically, (sub)cutaneous actinomycosis and nocardiosis are characterized by the presence of skin edema and inflammation. Ulceration and draining tracts are common.(10) In the thoracic form, the parietal and visceral pleura are inflamed and thickened by velvety proliferations. Similar lesions may be present within the mediastinum or pericardial sac. The thoracic cavity often contains a reddish-brown turbid effusion due to accumulation of sanguinopurulent fluid, which can cause compression atelectasis of the lungs.(3,5,13) Grossly, infected tissues might contain yellowish granules measuring about 0.1 cm in diameter (sulfur granules, tissue grains). Sulfur granules are common in actinomycosis, but rare in nocardiosis.(3,9,10) Occasionally sulfur granules might be observed in infection with N. caviae and N. braziliensis, whereas they are usually absent in infection caused by N. asteroides.(10)
The microscopic hallmark of nocardiosis and actinomycosis is pyogranulomatous inflammation with intralesional large colonies of filamentous bacteria.(5) If the infection exists over a longer time, granulation tissue formation, fibrosis and/or infiltration with lymphocytes and plasma cells is usually present. A modified acid-fast stain (e.g. Fite-Faraco modification) will stain Nocardia spp., but not Actinomyces spp.(3) On cytological and histopathological examination, the sulfur granules are composed of large bacterial colonies surrounded by eosinophilic clubbed material. The eosinophilic clubbed material is considered to be caused by an antigen-antibody reaction (Splendore-Hoeppli reaction).(6,9,10)
Proliferative pleuritis with reddish-brown turbid thoracic effusion is diagnostic for actinomycosis and nocardiosis. Skin lesions of actinomycosis and nocardiosis have to be differentiated from bacterial pseudomycetoma, which is also characterized by skin edema and pyogranulomatous inflammation. Ulceration, draining tracts and tissue grains might be present as well. In contrast to actinomycosis and nocardiosis, the intralesional bacterial colonies in pseudomycetoma are composed of non-filamentous Gram-positive or Gram-negative bacteria surrounded by Splendore-Hoeppli reaction. Possible causes of bacterial pseudomycetoma are Staphylococcus, Streptococcus, Proteus spp. and Pseudomonas spp.(10)
An incidental finding in this case, unrelated to the actinomycosis, was the presence of megakaryocytes in some pulmonary capillaries. Megakaryocytes can occasionally be observed within pulmonary capillaries of different animal species. It has been shown that megakaryocytes can exit the intact bone marrow and arrest in pulmonary capillaries, where they can release platelets in the circulation.(14)
JPC Diagnosis:
Conference Comment:
References:
2. Barnes LD, Grahn BH: Actinomyces endophthalmitis and pneumonia in a dog. Can Vet J 48:1155-1158, 2007
3. Biberstein EL, Hirsh DC: Filamentous bacteria: Actinomyces, Nocardia, Dermatophilus, and Streptobacillus. In: Veterinary Microbiology, eds. Hirsh DC, MacLachlan NJ, Walker RL, 2nd ed., pp. 215-222. Blackwell Publishing, Oxford, UK, 2004
4. Brown CC, Baker DC, Barker IK: Alimentary system. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 1-296. Saunders, Elsevier, Philadelphia, PA, 2007
5. Caswell JL, Williams KJ: Respiratory system. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 523-653. Saunders, Elsevier, Philadelphia, PA, 2007
6. Couto SS, Dickinson PJ, Jang S, Munson L: Pyogranulomatous meningoencephalitis due to Actinomyces sp. in a dog. Vet Pathol 37:650-652, 2000
7. Edwards, DF: Actinomycosis and Nocardiosis. In: Infectious Diseases of the Dog and Cat, ed. Greene CE, 2nd ed., pp. 303-313. W.B. Saunders Company, Philadelphia, PA, 1998
8. Hirai T, Nunoya T, Azuma R: Actinomycosis of the brain and temporal bone in a goat. J Vet Med Sci 69:641-643, 2007
9. Ginn PE, Mansell JEKL, Rakich PM: Skin and appendages. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 553-781. Saunders, Elsevier, Philadelphia, PA, 2007
10. Gross TL, Ihrke PJ, Walder EJ, Affolter VK: Infectious nodular and diffuse granulomatous and pyogranulomatous diseases of the dermis. In: Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis, eds. Gross TL, Ihrke PJ, Walder EJ, Affolter VK, 2nd ed., pp. 272-319. Blackwell Publishing, Oxford, UK, 2005
11. Ledbetter EC, Scarlett JM: Isolation of obligate anaerobic bacteria from ulcerative keratitis in domestic animals. Vet Ophthalmol 11:114-122, 2008
12. Pelle G, Makrai L, Fodor L, Dobos-Kov+�-�cs M: Actinomycosis of dogs caused by Actinomyces hordeovulneris. J Comp Pathol 123:72-76, 2000
13. Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC: Pathogenic bacteria. In: Veterinary Microbiology and Microbial Disease, eds. Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC, pp. 43-215, Blackwell Publishing, London, UK, 2002
14. Zucker-Franklin D, Phillipp CS: Platelet production in the pulmonary capillary bed. Am J Pathol 157:69-74, 2000