CASE IV: 18N045 (JPC 4160777)
Signalment:
6 to 8-week-old female athymic nude mouse (Mus musculus, Foxn1nu, background strain not reported)
History:
This athymic nude mouse had been injected subcutaneously with a human-derived tumor line by an approved rodent vendor. Several weeks later, the mouse was delivered to our facility. One week after delivery the mouse was reported for moderate to severe scaly dermatitis. No other clinical signs were reported.
Gross Pathology:
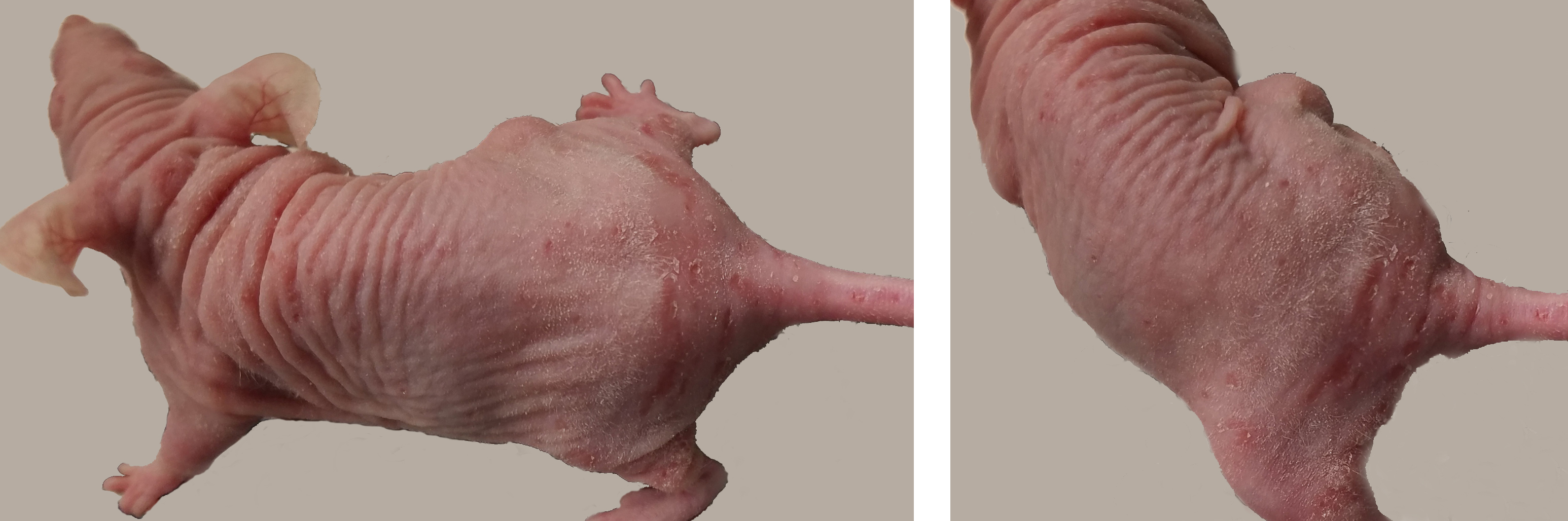
Skin: There was moderate ulcerative and proliferative dermatitis extending over most of the dorsum, hind limbs, and tail. Ulcers were most severe at the base of the tail (photos). There was an approximately 1 cm subcutaneous mass on the right flank (presumed to be the injected tumor). Because C. bovis was the presumed etiologic agent, the mouse was euthanized.
Laboratory results:
Skin swabs samples were PCR positive for Corynebacterium bovis.
Microscopic Description:
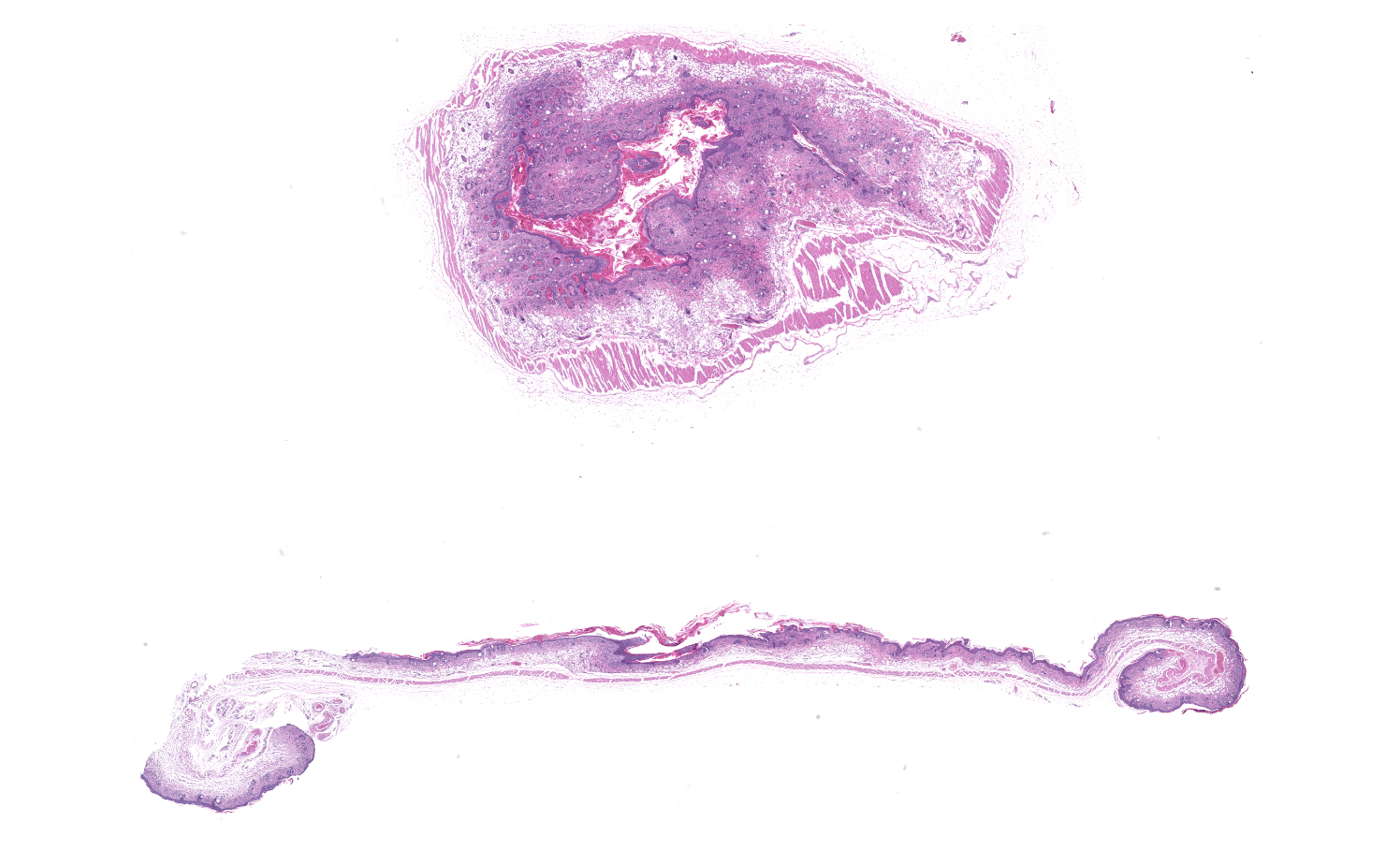
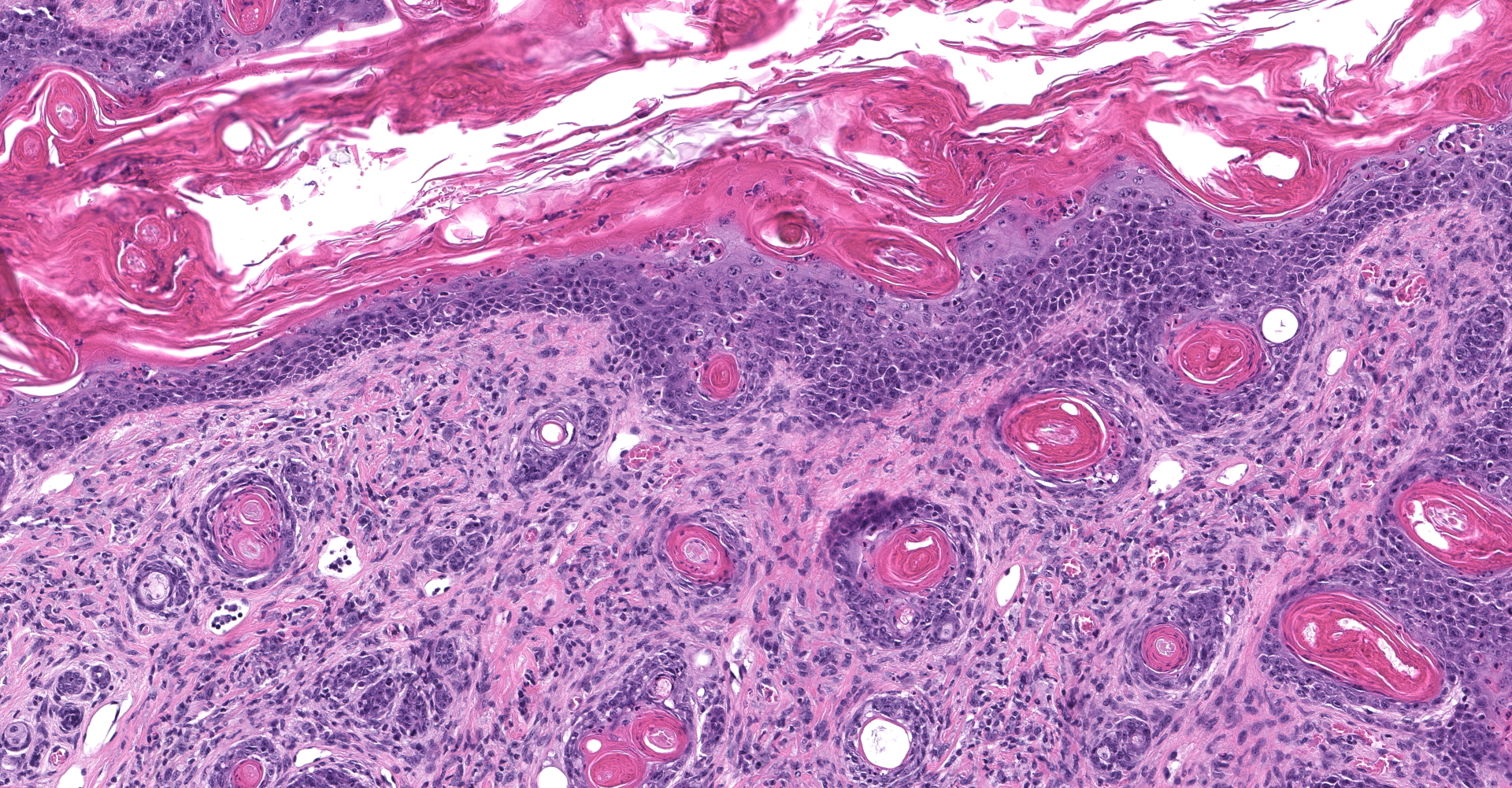
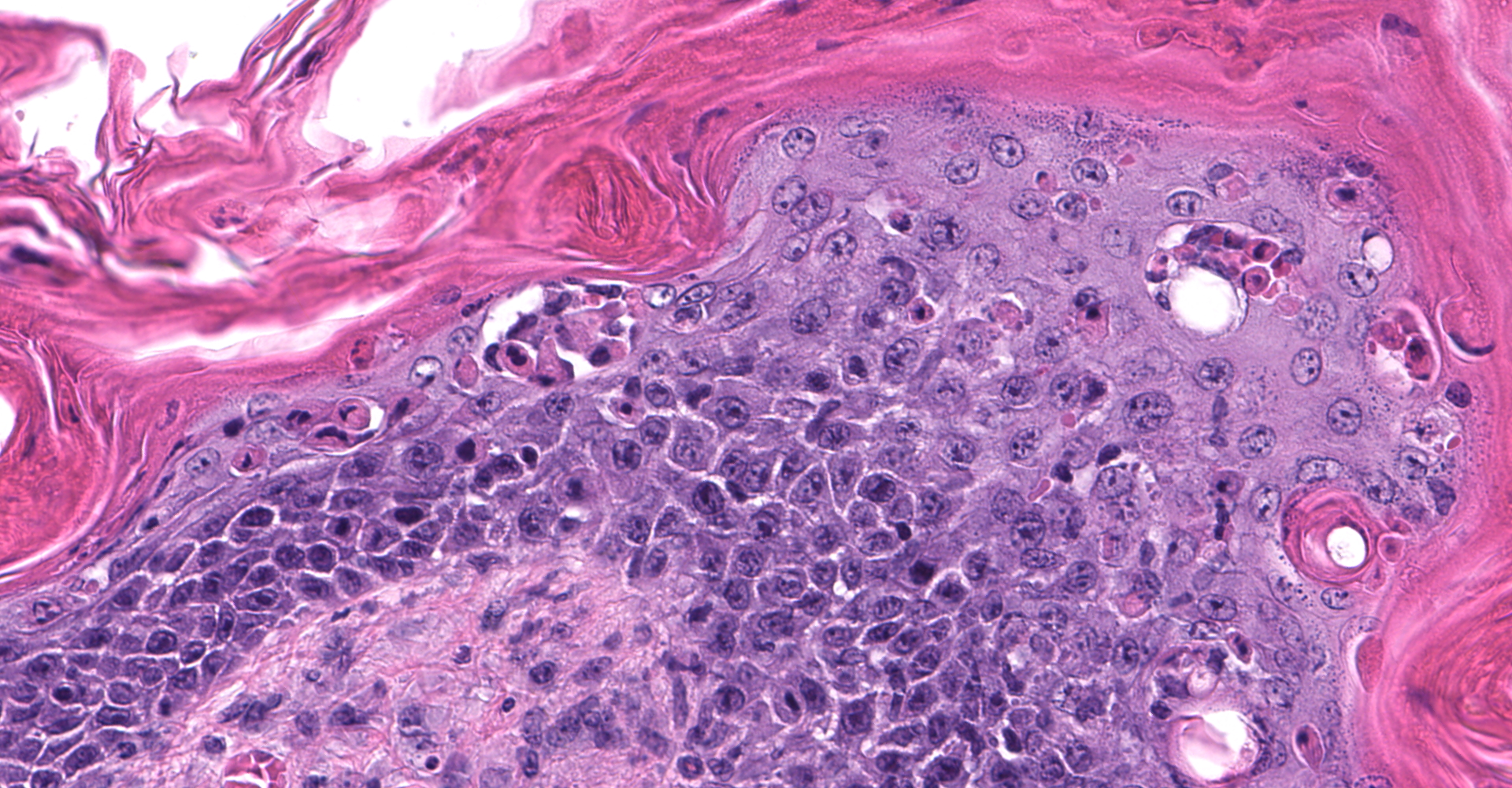
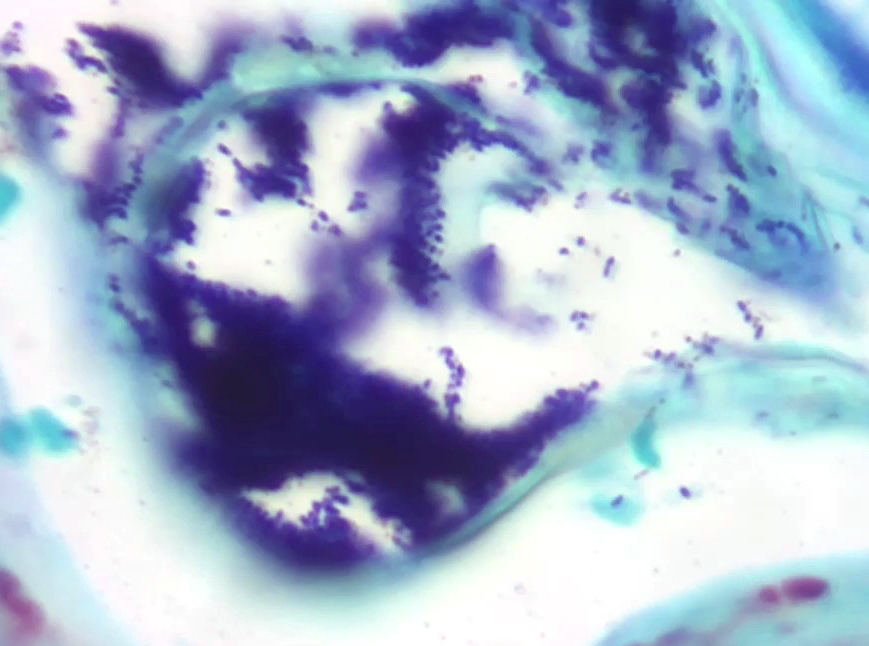
Skin: Severe widespread neutrophilic dermatitis, characterized by marked transdermal neutrophilic inflammation and multifocal epidermal necrosis. There is marked widespread hyper- and parakeratosis with scattered intraepidermal clefts, some of which contain large numbers of acantholytic cells, and/or neutrophils. The epidermis is hyperplastic. There is widespread single-cell necrosis as well as marked disorganization of the epidermal layers. Hair follicles and surface epidermis are both affected.
A Gram stain revealed clusters of gram-positive cocci within the keratin layers with equivocal short gram-positive rods.
Contributor's
Morphologic Diagnoses:
Dermatitis:
Chronic, severe, neutrophilic with intradermal clefts and abscesses,
acantholytic cells, hyper-and parakeratosis, and gram-positive cocci.
Contributor's Comment:
Upon gross examination, the dermatitis in this mouse was initially presumed to be due to Corynebacterium bovis, consistent with the PCR results. C. bovis is a common colonizer of the skin in laboratory mice, and is associated with hyperkeratosis, parakeratosis, and epidermal hyperplasia with minimal to absent inflammation. The gross lesion is particularly apparent in nude mice because of the absence of a normal hair coat, but other immunosuppressed mouse strains as well as immunocompetent strains can also be colonized and develop lesions.1 When it was first identified as the cause of scaly skin disease (also called Corynebacterium-associated hyperkeratosis) in nude mice, it was considered a pathogen of high morbidity but low mortality5, but in recent years, infection with C. bovis has been associated with research complication including immunological anomalies, wasting disease, and failure of engraftment of tumor cells.6 For this reason, some institutions exclude this organism, and PCR detection sometimes leads to depopulation of research colonies.
In the present case, histologic examination revealed severe inflammatory lesions that are not characteristic of C. bovis colonization of mice. Severe inflammation with ulceration, intradermal abscesses, crusting, and other damage to the epidermis and dermis are not usual findings. In addition, the presence of colonies of gram-positive coccoid bacteria in the keratin layer were suggestive of infection with Staphylococcus spp. The lesions in this case are similar to those described by Russo et al.4 in which an inflammatory dermatitis in a nude mouse was associated with culture of Staphylococcus xylosus. In that report, lesions were similar to the current case, with individual keratinocyte necrosis, ulceration, and crust formation, but intracorneal pustules and acantholytic cells were not described. C. bovis was not isolated in that case, but large numbers of Staphylococcus xylosus were isolated and clusters of gram-positive cocci in the keratin layer were demonstrated in histologic sections of skin. Based on the bacteriologic and histologic findings, a diagnosis of dermatitis due to S. xylosus was made. Like the current case, the published case was a single mouse in a group of unaffected mice. In that report, PCR was not performed.
The causative or contributory role of C. bovis in our case was complicated by the unusual histologic features and the absence of bacteriologic confirmation. Simple C. bovis colonization is associated with mild hyperkeratosis and scattered short gram-positive rods that in the keratin layer. Inflammation is minimal or not present. In this case, there was marked inflammation with some features of immune-mediated disease, including acantholytic cells both within intracorneal pustules and individually within the stratum spinosum, and disruption of the stratum basale. Gram staining revealed clusters of gram-positive cocci, most consistent with Staphylococcus sp. Whether or not C. bovis contributed to the lesions in this mouse could not be established. However, the ulcerations noted grossly and the atypical inflammatory and necrotizing lesions in the skin suggested that a different or additional infectious agent was present. Unfortunately, the skin was not cultured, and the presence or absence of viable C. bovis or other bacteria could not be established.
Contributing Institution:
In Vivo Animal Core, Unit for Laboratory Animal Medicine
University of Michigan Medical School
https://ncrc.umich.edu/research/scientific-resources/ivac
JPC
Diagnosis:
Haired skin: Dermatitis, neutrophilic and histiocytic, perivascular, moderate, with hyperkeratosis, acanthosis, apoptotic keratinocytes, and intracorneal bacilli and cocci.
JPC Comment:
As noted by the contributor, Corynebacterium bovis presents a significant challenge to in the research setting. As an example, a pre-clinical research laboratory was recently affected by broad dissemination of C. bovis, resulting in cutaneous infections in murine inventories that diminished patient-derived chronic myelomonocytic leukemia engraftment in immunodeficient mice, resulting in reduced confidence in preclinical data validity. In an effort to exclude this organism and reduce risk of such invalidations, many institutions conduct facility-wide C. bovis PCR murine and environmental surveillance, cull positive mice, and sterilize of equipment and rooms via methods such as vaporized hydrogen peroxide.2
C. bovis is an opportunistic, lipophilic, gram-positive coryneform rod often found within the stratum corneum. The organism resides of the skin of mice, particularly affecting immunodeficient strains such as nu/nu, Prkdcscid, SCIDbeige, and others. Persistent infections result in the development of an orthokeratotic, hyperkeratotic, acanthotic dermatitis that subsequently generates C. bovis-infected keratin flakes that can be spread by airborne and fomite transmission within facilities.2,3
The exact mechanism by which C. bovis colonizes murine skin and induces the previously described lesions has not been described. Historically, immunocompetent and immunodeficient mice have been known to be vulnerable to transient infection but only immune deficient strains, such as nude and SCID strains, were thought to develop "persistent" infections that progress into clinically apparent hyperkeratosis following natural infection. However, a reported natural outbreak of C. bovis in a laboratory resulted in persistent infections in not only immunodeficient but also some immunocompetent strains, including transgenic mice expressing the human papillomavirus (HPV) E6 oncoprotein and epidermal mutant dep/dep mice. Epidermal mutant dep/dep mice remained persistently PCR positive for >45 days and developed clinically apparent hyperkeratosis.2
Corynebacterium species cannot produce their own lipids and are well suited to reside within lipid-rich sebum and the stratum corneum. Therefore, a possible explanation for persistent infections within the immunocompetent dep/dep strain was the presence of excessive sebum from hyperplastic sebaceous glands and abundant lipids of the interfollicular epidermis which created a favorable environment for the organism's survival.2
Cutaneous dysbiosis with an overrepresentation of Corynebacterium species as a result of altered skin homeostasis has also been reported in mice. Therefore, a possible explanation for persistent infection in immunocompetent transgenic mice that express the HPV-E6 oncoprotein may be due to altered skin homeostasis as the result of a human keratin 14 promoter directed E6 oncogene expression in the hair follicle and epidermal basal layer, resulting epidermal hyperplasia.2
Definitive diagnosis of Corynebacterium bovis can be achieved through culture or PCR of skin swabs or feces, although the organism can be isolated from the oral cavity, skin, and heart blood of infected mice. Cultures should be held for up to seven days due to the organism's slow growth.3
Differential diagnoses include low ambient humidity in addition to Staphylococcus xylosus, as previously described by the contributor in a recent report.
Both Staphylococcus aureus and S. xylosus are known to cause another syndrome in mice known as ulcerative dermatitis.3 Some strains, such as B6 and manifest excessive scratching behavior which facilitates colonization, most often by S. aureus. Similarly, athymic nude mice and B6-Nos2tm1Lau (NOS2) strains are affected by S. xylosus. Following colonization, the bacteria produce virulence factors such as hemolysis, nucleases, proteases, lipases, hyaluronidase, collagenase, and exotoxins that result in focal small to large chronic ulcerative lesions, most often around the head and neck, but also on the trunk and tail base. Histologically, these infections are characterized as prominent colonies of gram-positive cocci within a superficial exudate and coagulative necrosis of the underlying epidermis and dermis which resemble 1st, 2nd, and 3rd degree burns with varying degrees of leukocytic infiltration and granulation tissue.3
Conference participants agreed with the contributor's assessment that the histologic features observed in this case were atypical for simple C. bovis colonization and suspect an additional etiologic agent, such as Staphylococcus spp. contributed toward this lesion's pathogenesis. As previously noted, C. bovis is typically associated with mild hyperkeratosis with minimal to mild inflammation in contrast to the robust inflammation observed in this case.
References:
1. Gobbi A, Crippa L, Scanziani E. Corynebacterium bovis infection in immunocompetent hirsute mice. Lab Anim Sci. 1999;49(2):209-211.
2. Miedel EL, Ragland NH, Slate AR, Engelman RW. Persistent Corynebacterium bovis Infectious Hyperkeratotic Dermatitis in Immunocompetent Epidermal-Mutant dep/dep Mice. Vet Pathol. 2020;57(4):586-589.
3. Percy DH, Barthold SW. Mouse. In: Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and 4th ed. Ames, IA: Blackwell Publishing; 2016:67-71.
4. Russo M, Invernizzi A, Gobbi A, Radaelli E. Diffuse scaling dermatitis in an athymic nude mouse. Veterinary Pathology. 2013;50(4):722-726. doi:10.1177/0300985812463408.
5. Scanziani E, Gobbi A, Crippa L, et al. Outbreaks of hyperkeratotic dermatitis of athymic nude mice in northern Italy. Laboratory Animals. 1997;31(3):206-211. doi:10.1258/002367797780596310.
6. Vedder AR, Miedel EL, Ragland NH, et al. Effects of Corynebacterium bovis on Engraftment of Patient-derived Chronic-Myelomonocytic Leukemia Cells in NSGS Mice. Comp Med. 2019;69(4):276-282. doi:10.30802/AALAS-CM-18-000138.