CASE IV: 19-355 (JPC 4161131)
Signalment:
3-month-old, castrated male, Holstein-Friesian ox (Bos taurus)
History:
The calf arrived at the barn late November as part of an artificial heart preclinical trial. On arrival, it had a dry cough and wheezes in the right cranioventral lung, but no ocular or nasal discharge. The calf had received a dose of tildipirosin antibiotic from the vendor. Later that afternoon, the staff noticed the calf appeared to have a weird breathing pattern and was using abdominal effort, so the calf was placed on report, and a veterinarian checked on it once a day. It did have an abdominal effort to its breathing, but the calf's respiratory rate was within normal limits, with a normal temperature. There was no change in the severity of the wheezes. One week after arrival, the calf received a dose of ceftiofur antibiotic to the base of its left ear and was moved from the receiving barn to the calf room. Two days later, the calf was put under isoflurane anesthesia to receive bilateral jugular catheters and remove the growth hormone implant in its right ear in preparation for the longer artificial heart implantation surgery. Induction and anesthesia went smoothly. However, it took a while for the calf to breathe on its own so that it could be extubated. When the calf was extubated, there was a brief period where it seemed to be in respiratory distress, despite pulse oximeter oxygen levels within normal limits. When the calf was standing on its own, it was taken back to the calf room, where it ate grain. About an hour later, the calf began to show signs of respiratory distress (open mouth breathing, increased abdominal effort). The calf was given supplemental oxygen through a nasal cannula, and blood gas showed severe respiratory acidosis. It was administered intravenous furosemide and steroids. The calf did not show any significant improvement with treatment and was a poor surgical candidate. The laboratory elected humane euthanasia.
Gross Pathology:
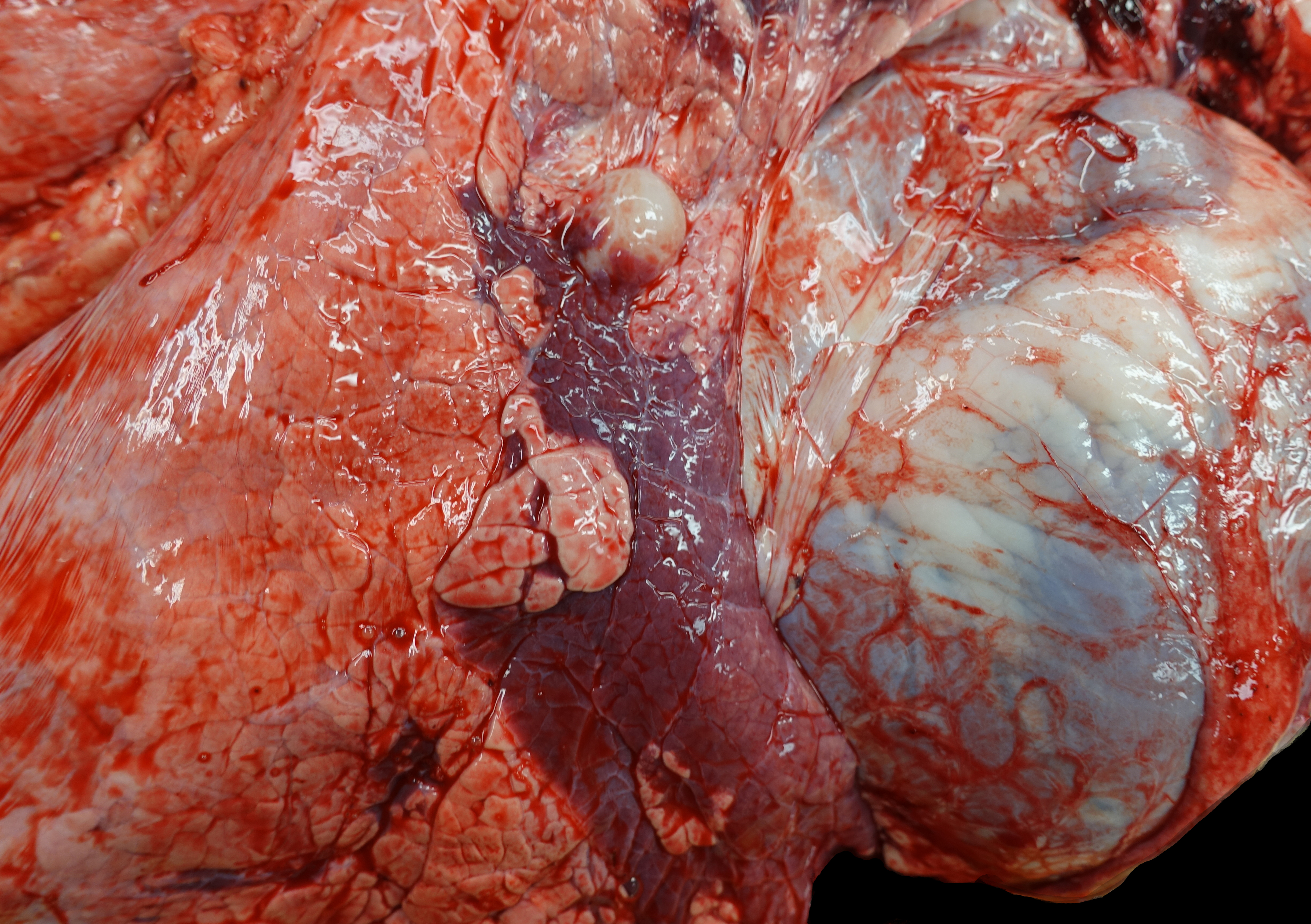
Upon opening the chest, a 2.5x3x1.5 cm region in the cranial aspect of the caudal left lung lobe is sharply and distinctly dark red and depressed (atelectasis). Up to 20% of the right cranial and middle lung lobes are also dark red and consolidated. When bisected, the consolidated tissue has a faint tan-lobulated pattern. A tracheobronchial lymph node near the region of lung consolidation on the right side is 1.7 mm-diameter and dense. The trachea diffusely contains adherent and free strands of tan fibrillary material and has a roughened surface.
The cranial pole of the right kidney contains two multiloculated, yellow translucent fluid-filled, thin-walled spaces (renal cysts) concentrated on the renal pelvis/collecting ducts of the affected lobules. The first is 5x5x4.5 cm, and the second is 2x1x1 cm. The left kidney and remaining organs are grossly within normal limits. The rumen contains hay and less abundant grain. The intestines are filled with green-brown digesta.
Laboratory results:
Bacterial culture:
Aerobic bacterial culture of the tracheal mucosa failed to grow bacteria.
Virus isolation:
The lung was positive for bovine coronavirus (BCoV) but negative for bovine parainfluenza 3 (PI3), bovine respiratory syncytial virus (BRSV), and infectious bovine rhinotracheitis virus (IBR)/bovine herpes virus (BHV1).
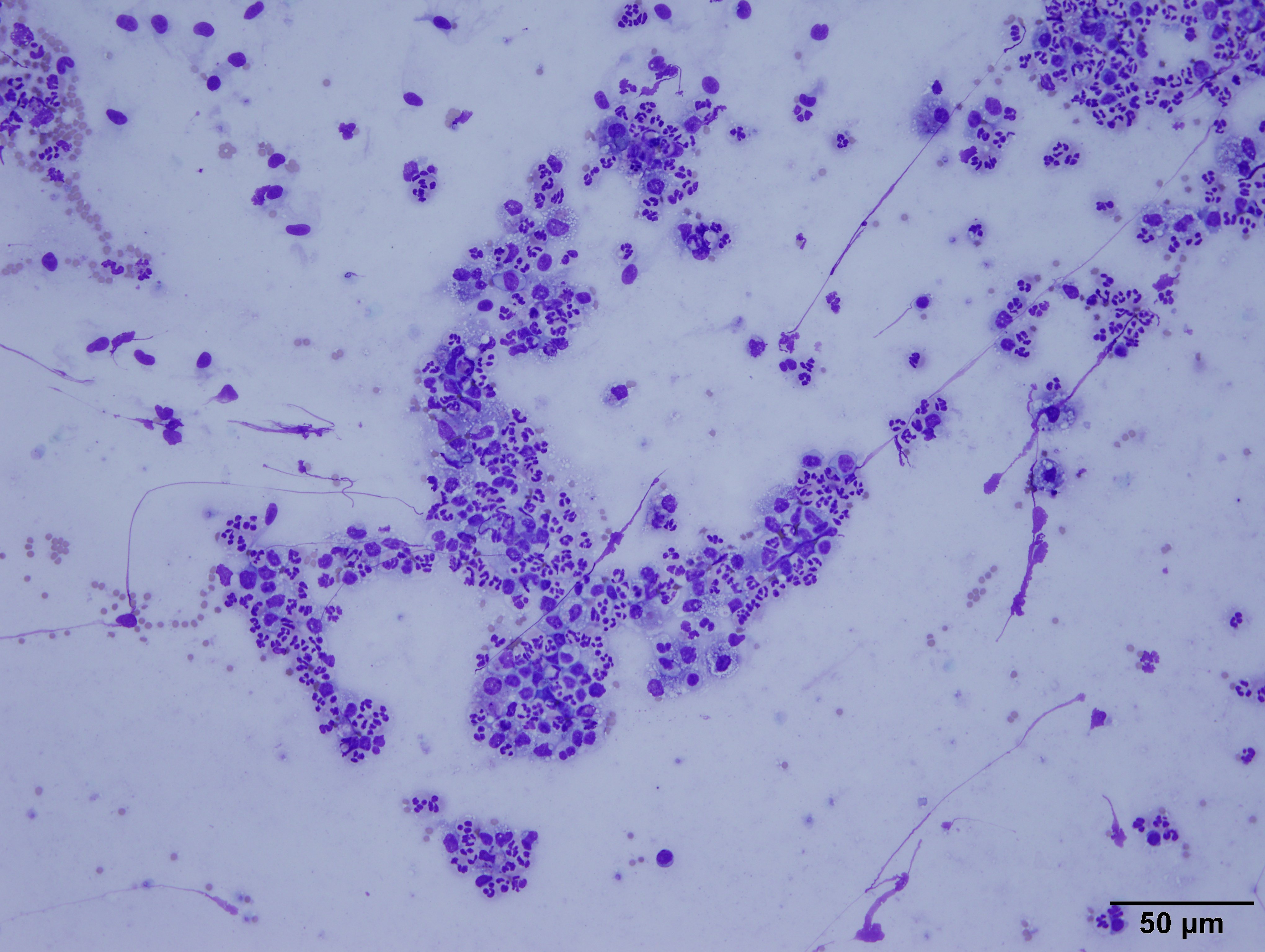
Cytology:
The highly cellular slide was stained with Diff-Quik. It contained
myriads of viable and degenerate neutrophils, eosinophilic cellular debris,
abundant light blue streaming material (mucous), and many alveolar macrophages
on a background of numerous red blood cells (peripheral blood). The macrophages
often had basophilic, foamy cytoplasm and contained cellular debris. There were
rare, sloughed, ciliated bronchiolar epithelial cells.
Interpretation: Suppurative pneumonia
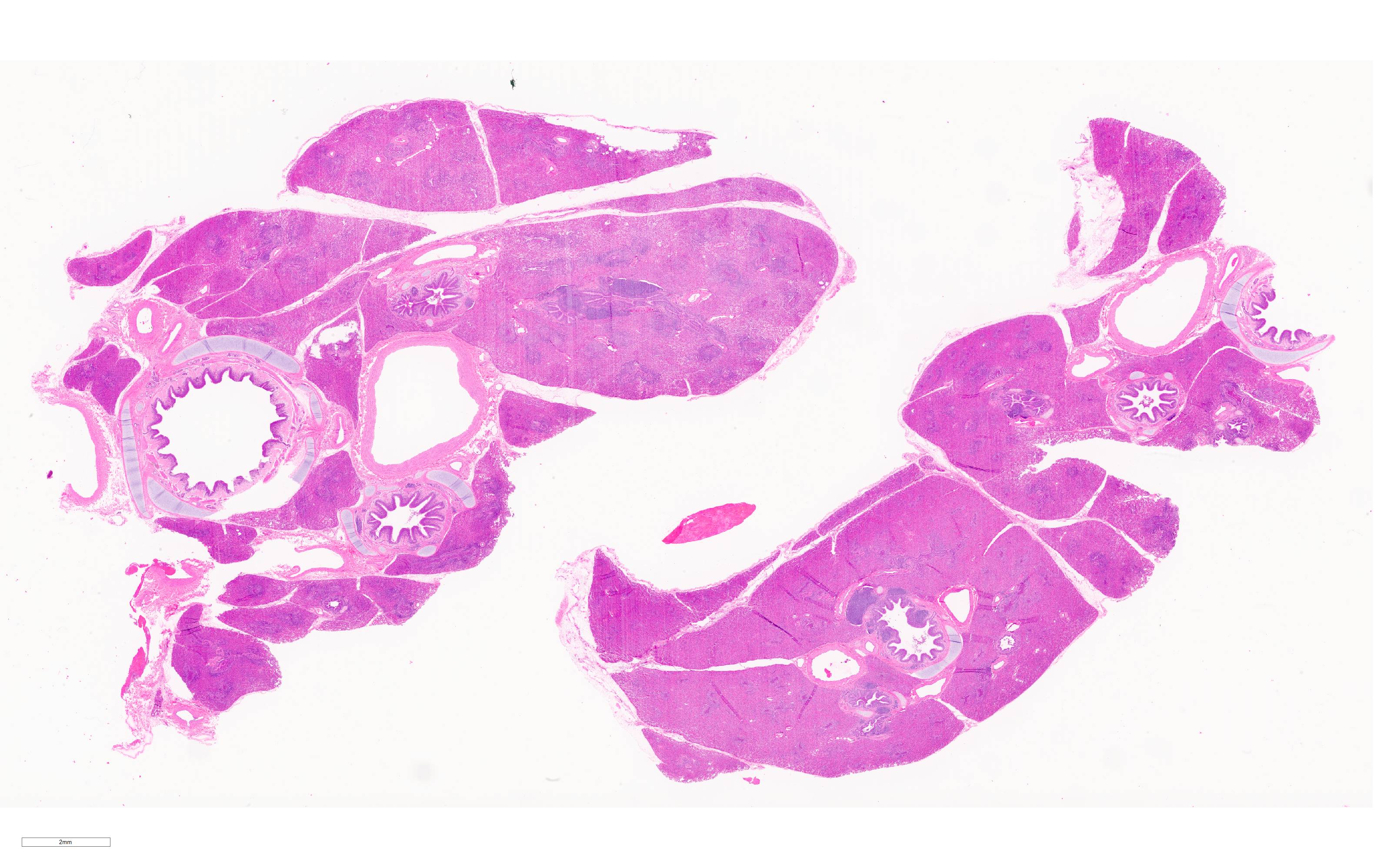
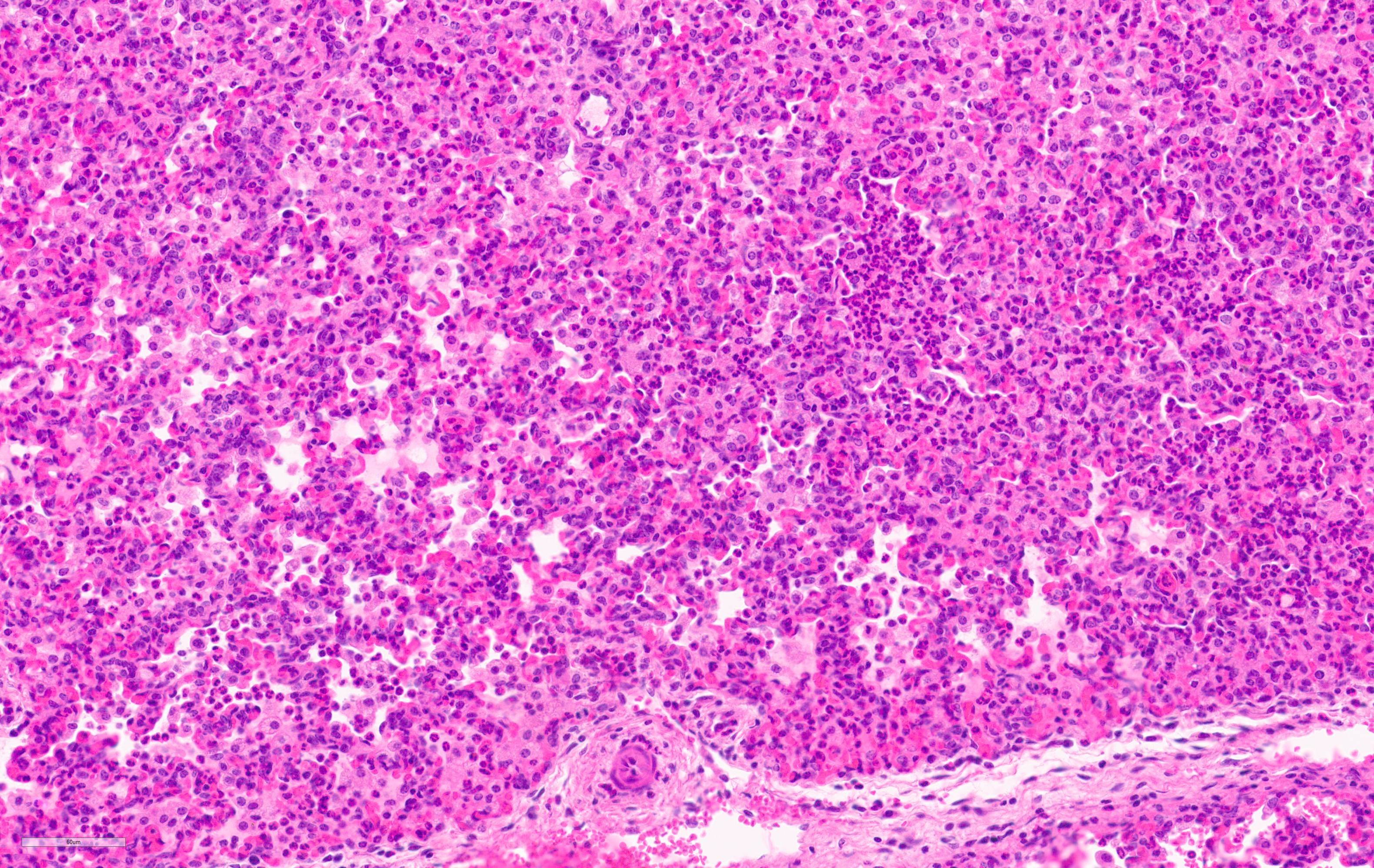
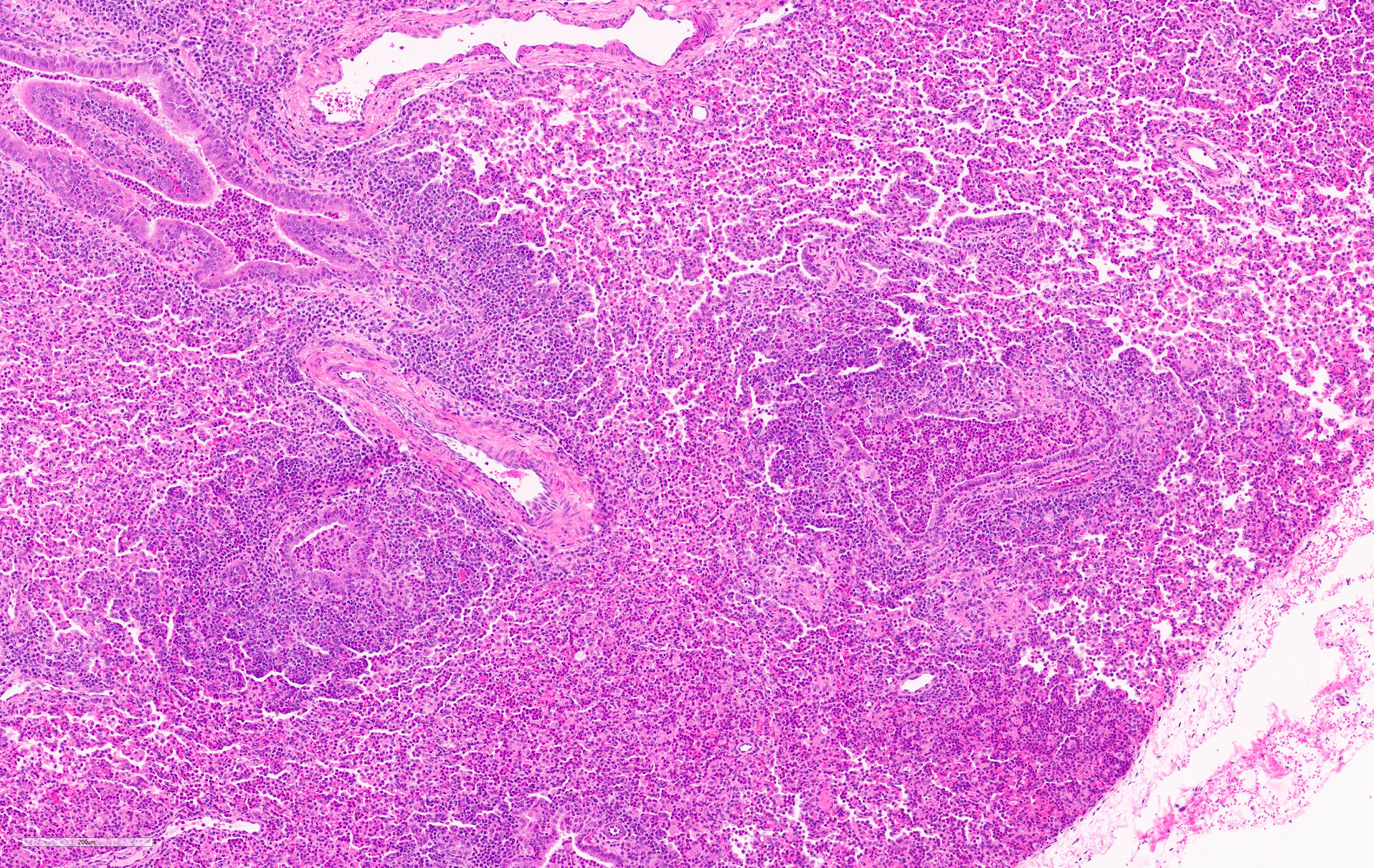
Microscopic Description:
Lung. 100% of the alveolar spaces are completely or partially collapsed, with the most intense regions surrounding bronchi or bronchioles (atelectasis). Airways contain abundant neutrophils and fewer macrophages along with eosinophilic cellular debris and degenerate epithelial cells that lack cytoplasmic and nuclear detail. The bronchiolar and bronchial epithelium contains dozens of scattered neutrophils and fewer macrophages that extend into the surrounding alveolar walls and alveolar spaces (bronchopneumonia). Peribronchiolar lymphoid tissue is prominent (lymphoid hyperplasia) throughout the section with additional clear space (edema), and abundant apoptotic lymphocytes and tingible body macrophages.
Contributor's Morphologic Diagnoses:
Lung: Bronchointerstitial pneumonia, regionally extensive, subacute, moderate-severe, suppurative with mild lymphoid hyperplasia.
Contributor's Comment:
Bovine coronavirus (BCoV) was isolated from the affected areas of lung. BCoV is a single-stranded, positive-sense RNA Betacoronavirus in the family Coronaviridae.5 It is an important cause of enteric disease in young calves that can also induce respiratory disease.1,4 BoCV is shed in feces and nasal secretions. Wildlife such as deer, waterbuck, elk, and water buffalo are possible reservoirs since they harbor coronaviruses that are closely related to BCoV.4 BCoV respiratory infections are exacerbated by stress or respiratory coinfections and may be observed with diarrhea.4 Respiratory disease outbreaks caused by BoCV are relatively common and mostly occur during winter months. A Belgium study found BoCV was one of the most frequently isolated respiratory viruses in young calves (38.4%), followed by bovine respiratory syncytial virus, and parainfluenza virus type 3.2 Coinfection of BCoV with bovine viral diarrhea virus or Histophilus somni may play an essential role in the pathogenesis of bovine respiratory disease.3,7 Therefore, the presence of BCoV likely increased the calf's susceptibility to developing suppurative pneumonia by damaging the tracheal epithelium and mucociliary apparatus. No bacteria were cultured from the tracheal swab. However, the calf had a dose of antibiotics three days before necropsy that may have decreased the bacterial load in the trachea. The enlarged lymph node near the condensed section of the right lung is suggestive of lymph node activation secondary to pneumonia. Additional differential diagnoses for BoCV respiratory infections in young calves include bovine parainfluenza 3 (PI3), bovine respiratory syncytial virus (BRSV), and infectious bovine rhinotracheitis virus (IBR)/bovine herpes virus (BHV1).
The renal cyst isolated to one pole of the kidney is considered an incidental finding. The cyst appeared to originate from the collecting duct or distal nephron based on its epithelial lining. No evidence of collecting duct obstruction was identified.1 The lymphocyte apoptosis is consistent with the dose of exogenous steroids administered before death during the period of respiratory distress. The localized infection of the skin and subcutaneous tissue at the base of the scrotum is common in calves castrated using elastic bands and would have healed with time and supportive care.
Contributing Institution:
Penn State College of Medicine, https://med.psu.edu/comparative-medicine
JPC Diagnosis:
Lung: Pneumonia, bronchointerstitial, suppurative and histiocytic, diffuse, severe, with marked BALT hyperplasia.
JPC Comment: The contributor provides a concise review of bovine coronavirus (BoCV), systems affected, and predisposing factors of this entity.
In addition to calf diarrhea and calf respiratory disease, BoCV is also associated with a third clinical syndrome known as "winter dysentery". Calf diarrhea caused by BoCV typically affects animals less than one month of age and is associated with declining maternal antibodies. Epithelial cells of the distal small and large intestines and colon are infected, resulting in villous atrophy and crypt hyperplasia. Following an incubation period of 3-4 days, calves develop severe malabsorptive diarrhea that persists for 2-8 days and associated with progressive dehydration, acidosis, hyperkalemia, hypoglycemia, and may progress to circulatory collapse and death. BoCV is interestingly found in both the intestinal and upper respiratory tract of most infected diarrheic calves at necropsy, consistent with concurrent fecal and nasal shedding. The disease is more prevalent in winter and tends to recur annually on the same farms, indicating reservoirs for infection within a herd may include subclinical infected calves or cows.4
Winter dysentery, in contrast to calf diarrhea, affects adult dairy and beef cattle as well as captive wild ruminants.4 This acute disease is characterized by hemorrhagic diarrhea, anorexia, and is predominantly seen in young postpartum dairy cows, resulting in significantly decreased milk production. 1,4 Similar to calf diarrhea, winter dysentery is also frequently associated with respiratory signs. Intestinal lesions are similar to those of calf diarrhea with high morbidity and low mortality rates of 20-100% and 1-2%, respectively.1,4 Despite the name "winter dysentery", this condition occurs in both cold and warm seasons.1
Respiratory disease caused by BoCV affects both calves (2-6mo) as well as young adult feedlot cattle (6-10mo). Uncomplicated cases typically exhibit mild respiratory disease, such as coughing and rhinitis. However, BoCV is recognized as one of many potential inciting factors in the development of bovine respiratory disease complex (BRDC), a multifactorial disease that predominately affects 6 to10-month-old feedlot cattle, also known as "shipping fever". The disease complex consists of interactions between viral, environmental, and host stress factors that create a permissive environment for bacterial infection, resulting in severe respiratory disease characterized by fever, dyspnea, and inflammatory and necrotizing pulmonary lesions leading to broncho(interstitial) pneumonia, weight loss, and often death.4 Etiologies associated with BRDC include, but are not limited to, viruses such as bovine respiratory syncytial virus, parainfluenza-3, bovine herpes virus-1 (infectious bovine rhinotracheitis), reovirus, rhinovirus, and BoCV, as well as the bacterial etiologies Histophilus somni, Mannheimia haemolytica, Pasteurella multocida, Bibersteinia trehalosi, Mycoplasma mycoides spp mycoides small colony type (contagious bovine pleuropneumonia), and Mycoplasma bovis.1
Given the ongoing Since the first human coronavirus (HCoV) was discovered in the 1960s within the nares of patients with the common cold, seven species have since been discovered in humans that lead to either mild or lethal respiratory disease depending on the strain type and patient's condition. HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 are typically associated with mild respiratory disease while SARS-CoV, MERS-CoV, and SARS-CoV-2 are associated with higher mortality rates (9.2%, 34%, and 2.9-12.6% respectively).6
These viruses typically originate in a natural host, such as a bat. However, an intermediate host is the typical source of zoonotic transmission. An intermediate host of SARS-CoV-2 has not yet been identified; however, analysis of samples obtained from Malytan pangolins in China indicate they are potential intermediate hosts for SARS-CoV-2.6
Although coronaviruses predominately cause respiratory disease in humans, different host receptors are targeted. As an example, SARS-CoV-2 first binds to ACE2 on the host cell surface through the S1 subunit and then fuses viral and host membranes through the S2 subunit, whereas MERS-CoV recognizes dipeptidyl peptidase 4 (DPP4; also known as CD26).6
It is unlikely SARS-CoV-2 will be the last novel coronavirus to infect humans given the trend observed since the 1960s. However, the robust research and scientific discovery associated with the current pandemic will contribute toward better understanding of novel species encountered in the future.6
References:
1. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier limited; 2016:148-150,537-546.
2. Pardon B, Callens J, Maris J, et al. Pathogen-specific risk factors in acute outbreaks of respiratory disease in calves. Journal of dairy science. 2020;103: 2556-2566.
3. Ridpath JF, Fulton RW, Bauermann FV, Falkenberg SM, Welch J, Confer AW. Sequential exposure to bovine viral diarrhea virus and bovine coronavirus results in increased respiratory disease lesions: clinical, immunologic, pathologic, and immunohistochemical findings. Journal of veterinary diagnostic investigation : official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 2020;32: 513-526.
4. Saif LJ, Jung K. Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. Journal of clinical microbiology. 2020;58.
5. Suzuki T, Otake Y, Uchimoto S, Hasebe A, Goto Y. Genomic Characterization and Phylogenetic Classification of Bovine Coronaviruses Through Whole Genome Sequence Analysis. Viruses. 2020;12.
6. Tang D, Comish P, Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16(5):e1008536. Published 2020 May 22.
7. Workman AM, Kuehn LA, McDaneld TG, Clawson ML, Loy JD. Longitudinal study of humoral immunity to bovine coronavirus, virus shedding, and treatment for bovine respiratory disease in pre-weaned beef calves. BMC veterinary research. 2019;15: 161.