WSC 22-23
Conference 9
Case II
Signalment:
10 year-old, male, castrated, German wirehaired pointer (Canis lupus familiaris)
History:
During the CT scan of the cervical vertebra, a mass in the thyroid was seen
Gross Pathology:
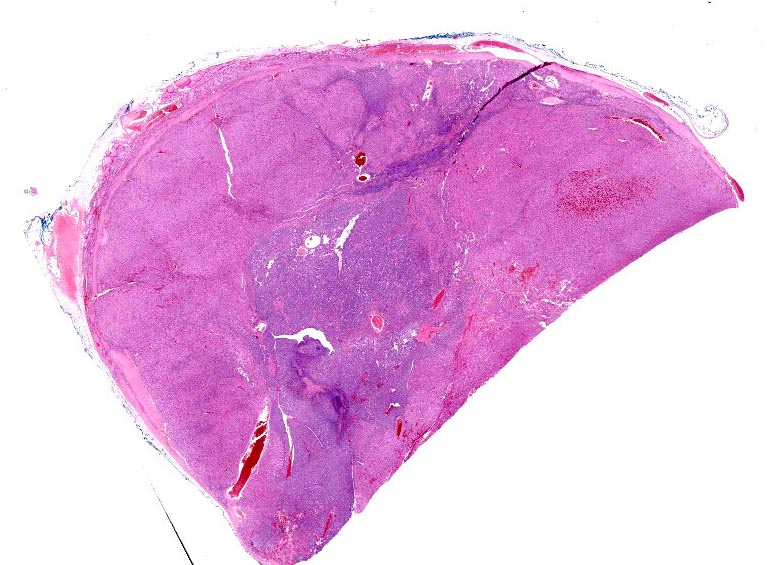
Excision of a beige brown egg-shaped tissue fragment measuring 3.3 x 2.1 x 1.9 cm. This one appears to be encapsulated. In cross section, the fragment has a beige brown aspect.
Laboratory Results:
None reported.
Microscopic Description:
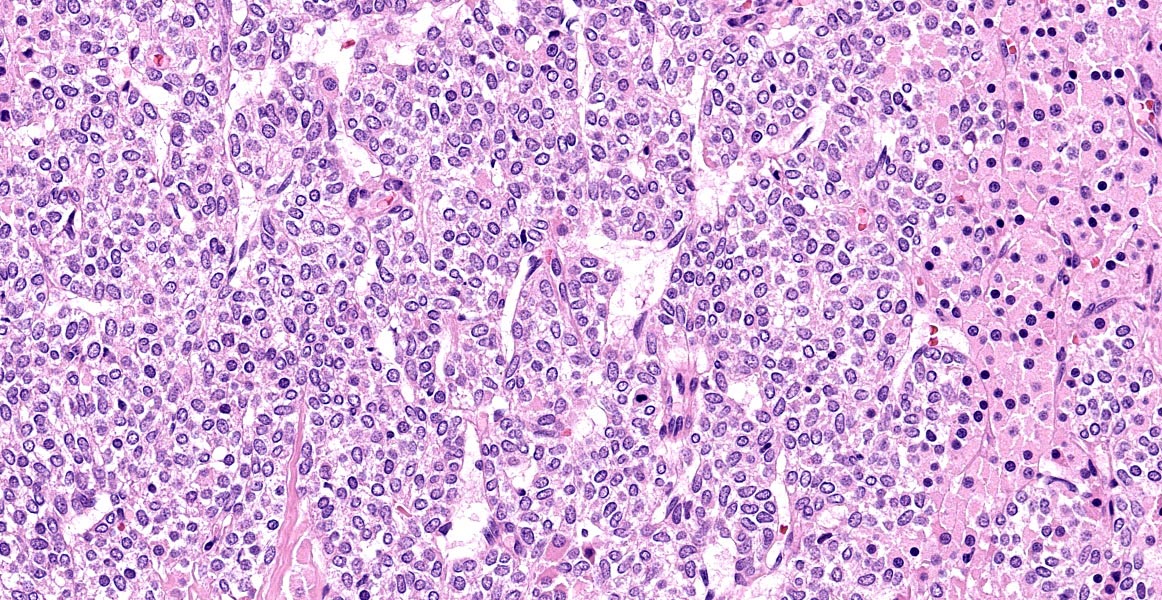
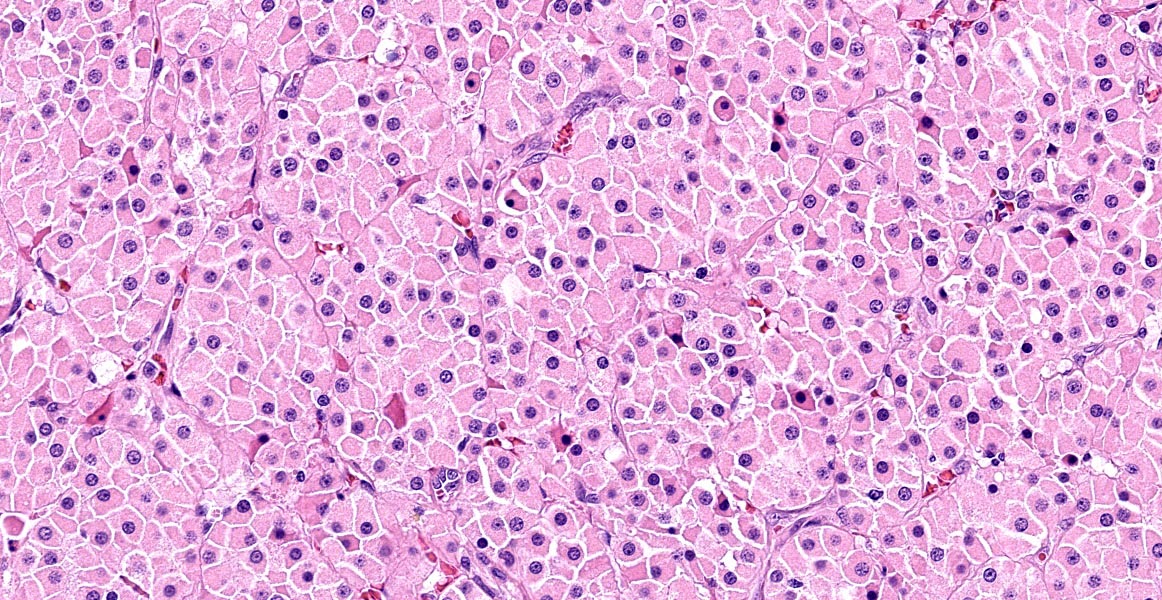
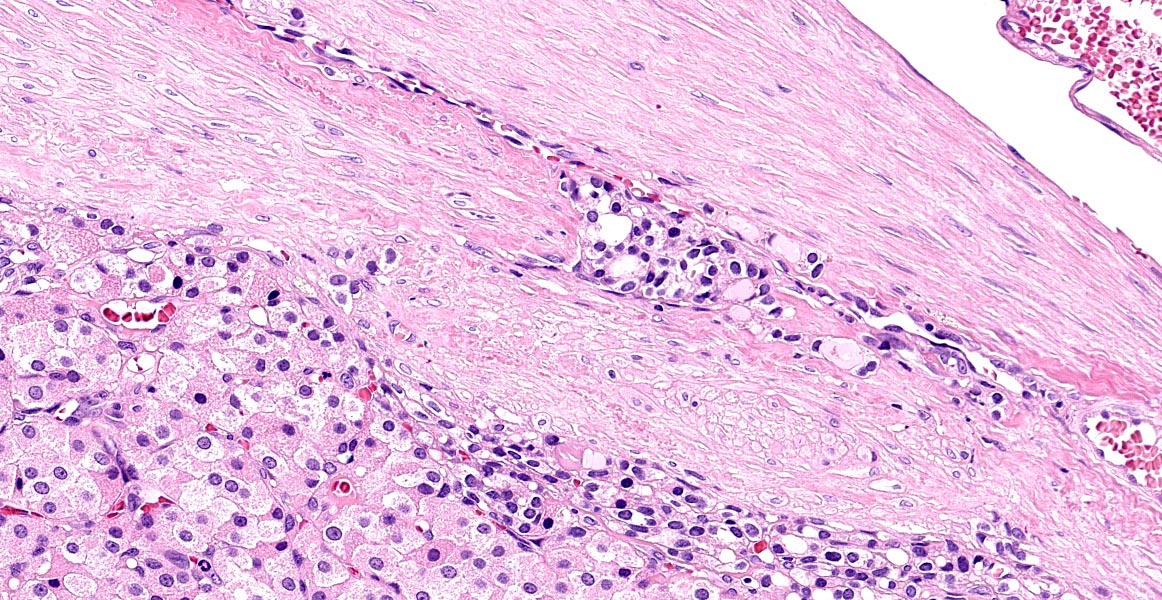
Thyroid gland: Predominantly solid, locally infiltrative and capsulated, there is a growing epithelial neoplasia, moderately cellular. Focally, the neoplastic infiltrate appears to extend into the surgical excision margin. The majority of the neoplastic cells grow in contiguous fields, nests and trabeculae and have a rounded to polygonal shape with a very substantial amount of eosinophilic and finely granular cytoplasm with a moderately sized, slightly varying rounded nucleus. In addition to the neoplastic cell component, varying sizes of fields and trabeculae, neoplastic cells with no apparent eosinophilia of the cytoplasm with predominantly larger, hypochromatic nuclei are also observed. In some places, both cell populations are found in close proximity to each other. In several areas, the neoplastic infiltrate shows growth through the surrounding connective tissue capsule with bulging fields of neoplastic cells in vascular lumens. In addition, in several places in the periphery, incisions of pre-existent, inactive thyroid tissue are seen. Mitoses are rare and there is mild anysokaryosis and anysocytosis.
Contributor’s Morphologic Diagnoses:
Thyroid, Hurtle-cell carcinoma
Contributor’s Comment:
Between 10 and 15% of the neoplasms located in the head and neck in dogs are originating from the thyroid3. Those neoplasms can be unilateral (most frequent) or bilateral and are often palpable near the larynx as a firm or soft mass.8,10 Most thyroid carcinomas are nonfunctional8. Thyroid carcinoma is the most common and most frequently diagnosed15 endocrine malignancy in dogs8 representing 19% of thyroid tumors with up to 38% of metastasis, most of them are already present by the time of diagnosis15. Some breeds are thought to be predisposed as Boxers, Beagles, Siberian Huskies and Golden retrievers10,15 although in one study mixed breeds tend to be more affected.15 The age ranges from 9 up to 15 years8,15 without sex prevalence.10
Differences between carcinomas and adenomas are essentially based on the size of the neoplasm, adenoma being usually smaller. Therefore thyroid carcinomas are fixed due to the local invasion whereas thyroid adenomas are subcutaneously freely movable.10 Carcinomas are usually multinodular and often show central necrosis and hemorrhages.15 Early carcinomas tend to be well demarcated and later carcinomas lead to vascular invasion in 45% of the cases8, essentially into the branches of the cranial and caudal thyroid veins forming large tumor cell thrombi10 and resulting in an impossible surgical resection.8 Dogs and humans show similarities in the spontaneous development of thyroid carcinoma with metastases to the lungs although the incidence of metastases in dogs is higher by the time of the diagnosis.8 Risks of metastasis are likely to increase with the size of the neoplasm.8 Pulmonary metastases will often precede retropharyngeal and caudal cervical lymph nodes involvement8.
Thyroid tumors, in dogs, are classified according to the World Health Organization (WHO) based on the morphological features.7,15
Thyroid tumors can arise from either thyroid follicular cells or thyroid C-cells (parafollicular cells, chief cells). The latter cell type is considered to represent less than 5% of thyroid neoplasms in dogs.8
Thyroid follicular cell carcinomas are subdivided in different subtypes:
-- cells are forming follicular structures consisting of cubic or columnar epithelial with or without colloid3,7, the so-called compact cellular (solid) showing cells with centrally localized nuclei and pale eosinophilic and granular cytoplasm.
-- the presence of both follicular and solid areas refers to follicular-compact cellular carcinoma.3,7
-- papillary structures made of multiple lines of cubic cells extending into cystic spaces surrounded by fibrovascular stroma are so called papillary carcinoma.3,7
Follicular thyroid carcinoma can be also classified as well-differentiated, poorly differentiated, undifferentiated or carcinosarcoma3.
In the dog, tumors with granular cytoplasm staining brightly eosinophilic with hematoxylin and eosin (HE) include granular cell tumors, rhabdoid tumors, neuroendocrine tumors, and oncocytomas. Oncocytomas are rare, usually benign, tumors composed of oncocytes. Oncocytes2 also called Hurthle cells or oxyphilic cells, are large, polygonal cells originating from metaplastic transformation of mature glandular or non-glandular cells. The high number of mitochondria present give the appearance of the abundant eosinophilic cytoplasm. Additionally, the cells composing the neoplasm can show a granular eosinophilic cytoplasm will refer to the Hurthle cell tumour and are rarely seen in dogs.7 In the dog, oncocytomas have been reported originally in the larynx but also in the thyroid gland and in the kidney. Specific immunohistochemical markers are helpful in making a differential diagnosis. Oncocytomas specifically express cytokeratin. Additionally granular cell tumors are positive for vimentin and are negative for epithelial cell markers.2
This is a phenomenon of metaplasia that occurs in inflammatory disorders, such as thyroiditis, or other situations that result in cellular stress. The proliferation of oncocytes gives rise to hyperplastic and neoplastic nodules. Oncocytic cells in the thyroid are often called “Hürthle” cells; however, this is a misrepresentation because they were initially described by Askenazy, and the cells that Hürthle described were in fact C cells.1
Numerous studies indicated that the criteria that apply to follicular tumours of the thyroid also distinguish malignant from benign Hürthle cell lesions. These included capsular and vascular invasion.1
To differentiate follicular cell carcinoma from C-cell carcinoma immunohistochemistry (IHC) might be necessary. In general, C-cell thyroid carcinomas exhibit strong immunoreactivity to calcitonin, calcitonin gene-related peptide (CGRP), and napsin A or markers of neuroendocrine tissue (Hassan, Campos). While follicular cell thyroid carcinomas show positivity for thyroglobulin, Pax8, and thyroid transcription factor (TTF-1 or NKX2). With the Papanicolau stain, the cytoplasm may be orange, green, or blue. By electron microscopy, the cytoplasmic granularity is produced by large mitochondria filling the cell, consistent with oncocytic transformation.1
The metastatic rate of thyroid carcinoma in dogs is highly related to the volume of the tumor (>20 cm3), bilateral and cervical vascular invasion3. More recently other factors the presence of estradiol in cells expressing estrogen receptors15 and to a greater protein expression of factors related to proliferation and angiogenesis (TTF-1, PCNA and VEGF).13
Thyroid cancers in dogs can either be inherited or spontaneous. In some breeds as the Dutch German longhaired pointers, two deleterious recessive mutations in the TPO gene have been highly associated with the familial follicular cell carcinoma16. Other studies suggest that overexpression of VEGFR-1, VEGFR2, PDPK-1, AKT1, and AKT2 in canine follicular thyroid carcinoma and VEGFR-1, EGFR, and PIK3CA in canine medullary thyroid carcinoma suggests that the PI3K/Akt signaling pathway is activated and increased in the pathogenesis of thyroid cancer in dogs for both follicular thyroid carcinoma and medullary thyroid carcinoma. Missense mutations in K-RAS were occasionally identified in a follicular thyroid carcinoma and a medullary thyroid carcinoma which are likely to be relevant for thyroid gland tumorigenesis.4
In cats, thyroid carcinomas tend to be rare and occur much less frequently than adenomas or multinodular hyperplasia10. Metastases to regional lymph nodes and distant sites, unlike in dogs, are rarely reported in cats.8
Contributing Institution:
Informatie voor dierenartsen - Veterinair Pathologisch Diagnostisch Centrum - Universiteit Utrecht (uu.nl)
JPC Diagnosis:
Thyroid gland: Hurthle cell tumor.
JPC Comment:
The contributor provides a thorough review of thyroid neoplasia and a slightly more nebulous entity - the oncocytoma. The unique dual morphology of neoplastic cells drove spirited discussion amongst conference participants, with some initially considering the possibility of a collision tumor or parathyroid origin. A collision tumor was ruled out by the areas of gradual transition between the two morphologies and the lack of a capsule separating them. This week’s moderator, Dr. Donald Meuten, explained the criteria for lymphovascular invasion in the Veterinary Cancer Guidelines and Protocols reference guide: (1) intravascular tumor with a thrombus adhered, (2) neoplastic cells invading the wall and endothelium, (3) neoplastic cells surrounded by endothelium, and (4) neoplastic cells in a vessel confirmed by vascular immunohistochemistrical stains, with the first two criteria supported by more firm evidence in the literature. Traditionally, infiltration and capsular invasion have also been considered criteria of malignancy in thyroid follicular neoplasms. In this case, the moderator and conference participants did not favor a diagnosis of malignancy and opted to diagnose a tumor instead of a carcinoma.
As the contributor mentions, in the dog, oncocytomas have been reported in the larynx, thyroid, and kidney, and are most commonly found in the salivary gland.12 In cats, oncocytomas have been found in the salivary glands and periocular tissues.5 Additionally, there are rare reports in the nasopharynx and choroid plexus.5,6,17
Histologically, the main differential diagnoses for oncocytomas are granular cell tumors and rhabdomyomas. Granular cell tumors have been documented in the oral cavity, lung, pharynx, and brain, and are generally benign.11 Cytoplasmic granules in both oncocytomas and granular cell tumors are PAS positive; however, electron microscopy reveals them to be different structures; mitochondria in the former, and probably secondary lysosomes in the latter.11 Differentiation of rhabdomyomas may require immunohistochemical staining to confirm muscle origin.11 Another differential diagnosis in the kidney is a chromophobe renal cell carcinoma.11 This rare neoplasm is believed to originate from the intercalated cells of the collecting ducts; is negative for pancytokeratin and PAS; and is positive for vimentin, colloidal iron, and CD117.12
References:
- Asa SL. My Approach to Oncocytic Tumours of the Thyroid. J Clin Pathol. 2004; 57(3): 225-232.57, no. 3 (1 March 2004): 225-32.
- Buergelt CD, Adjiri-Awere A. Bilateral Renal Oncocytoma in a Greyhound Dog. Vet Pathol. 2020; 37(2):188-192.
- Campos M, Ducatelle R, Rutteman G, Kooistra HS, Duchateau L, de Rooster H, Peremans K, Daminet S. Clinical, Pathologic, and Immunohistochemical Prognostic Factors in Dogs with Thyroid Carcinoma. Jour Vet Intern Med. 2014;28(6):1805-1813.
- Campos M, Kool MMJ, Daminet S, Ducatelle R, Rutteman G, Kooistra HS, Galac S, Mol JA. Upregulation of the PI3K/Akt Pathway in the Tumorigenesis of Canine Thyroid Carcinoma. Jour Vet Intern Med. 2014; 28(6):1814-1823.
- Cossic B, Silver G, Kent M, et al. Surgical removal of a choroid plexus oncocytoma in an adult cat. J Small Anim Pract. 2017; 58(10):589-592.
- Doughty RW, Brockman D, Neiger R, McKinney L. Nasal Oncocytoma in a Domestic Shorthair Cat. Vet Pathol. 2006; 43:751-754.
- Gulcubuk A, Erdogan O, Bozkurt ER, Gurel A, Ar?kan N, Cat H. Case Report: Compact Cellular (Solid) Carcinoma Containing Hurthle Cell Areas in the Thyroid Gland of a Dog. Revue de Médecine Vétérinaire. 2013; 164(10). n.d.
- Hassan BB, Altstadt A, Dirksen WP, Elshafae SM, Rosol TJ. Canine Thyroid Cancer: Molecular Characterization and Cell Line Growth in Nude Mice. Vet Path. 2020;57(2):227-240.
- Kido N, Itagaki I, Ono K, Omiya T, Matsumoto R. FOLLICULAR CELL CARCINOMA OF THE THYROID GLAND IN THREE CAPTIVE AGED RACCOON DOGS ( NYCTEREUTES PROCYONOIDES ). J Zoo Wildl Med. 2015;46(4):889-894.
- Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Sixth edition. St. Louis, Missouri: Elsevier, 2016.
- Meuten DJ, Meuten TLK. Tumors of the Urinary System. In: Meuten DJ, ed: Tumors in Domestic Animals. 5th edition. Ames, IO: John Wiley & Sons, Inc. 2017; 640.
- Munday JS, Lohr CV, Kiupel M. Tumors of the Alimentary Tract. In: Meuten DJ, ed: Tumors in Domestic Animals. 5th edition. Ames, IO: John Wiley & Sons, Inc. 2017; 511-512.
- Pessina P, Castillo V, Sartore I, Borrego J, Meikle A. Semiquantitative Immunohistochemical Marker Staining and Localization in Canine Thyroid Carcinoma and Normal Thyroid Gland: Dog Thyroid Carcinoma Markers. Vet Comp Oncol. 2016; 14(3): e102-112.
- Ramos-Vara JA, Miller MA, Johnson GC, Pace LW. Immunohistochemical Detection of Thyroid Transcription Factor-1, Thyroglobulin, and Calcitonin in Canine Normal, Hyperplastic, and Neoplastic Thyroid Gland. Vet Pathol. 2002; 39(2):480-487.
- Soares LMC, Pereira AHB, de Campos CG, Rocha LS, dos Santos TA, Souza MA, Jark PC, Pescador CA. Histopathological and Immunohistochemical Characteristics of Thyroid Carcinoma in the Dog. J Comp Path. 2020; 177: 34-41.
- Yu Y, Bovenhuis H, Wu Z, Laport K, Groenen MAM, Crooijmans Richard PMA. Deleterious Mutations in the TPO Gene Associated with Familial Thyroid Follicular Cell Carcinoma in Dutch German Longhaired Pointers. Genes. 2021; 12(7): 997.
- You M, Kim Y, Woo G, et al. Nasopharyngeal oncocytoma in a cat. J Vet DIagn Invest. 23:391-394.