WSC 22-23
Conference 17
Case III:
Signalment:
Estimated 3-year-old, spayed-female, Beagle dog (Canis lupus familiaris)
History:
The patient was originally adopted from Puerto Rico and was estimated to be 3-years-old. The patient was reportedly up-to-date on routine veterinary care, including vaccination (last vaccination was given Sept 2018 - about 1 year prior to presentation).
Patient initially presented to the Neurology Service for a one-month history of progressive ataxia and lethargy. At that time, neurolocalization included the thalamic cortex and cerebellar vestibular system. MRI did not reveal any abnormalities and CSF analysis showed normal protein levels with a slightly elevated mononuclear cell count. Tick-borne disease panel was negative. The patient was managed over one month with doxycycline and corticosteroids. Over the following month, the patient continued to decline with progressive ataxia and mentation changes, resulting in euthanasia.
Gross Pathology:
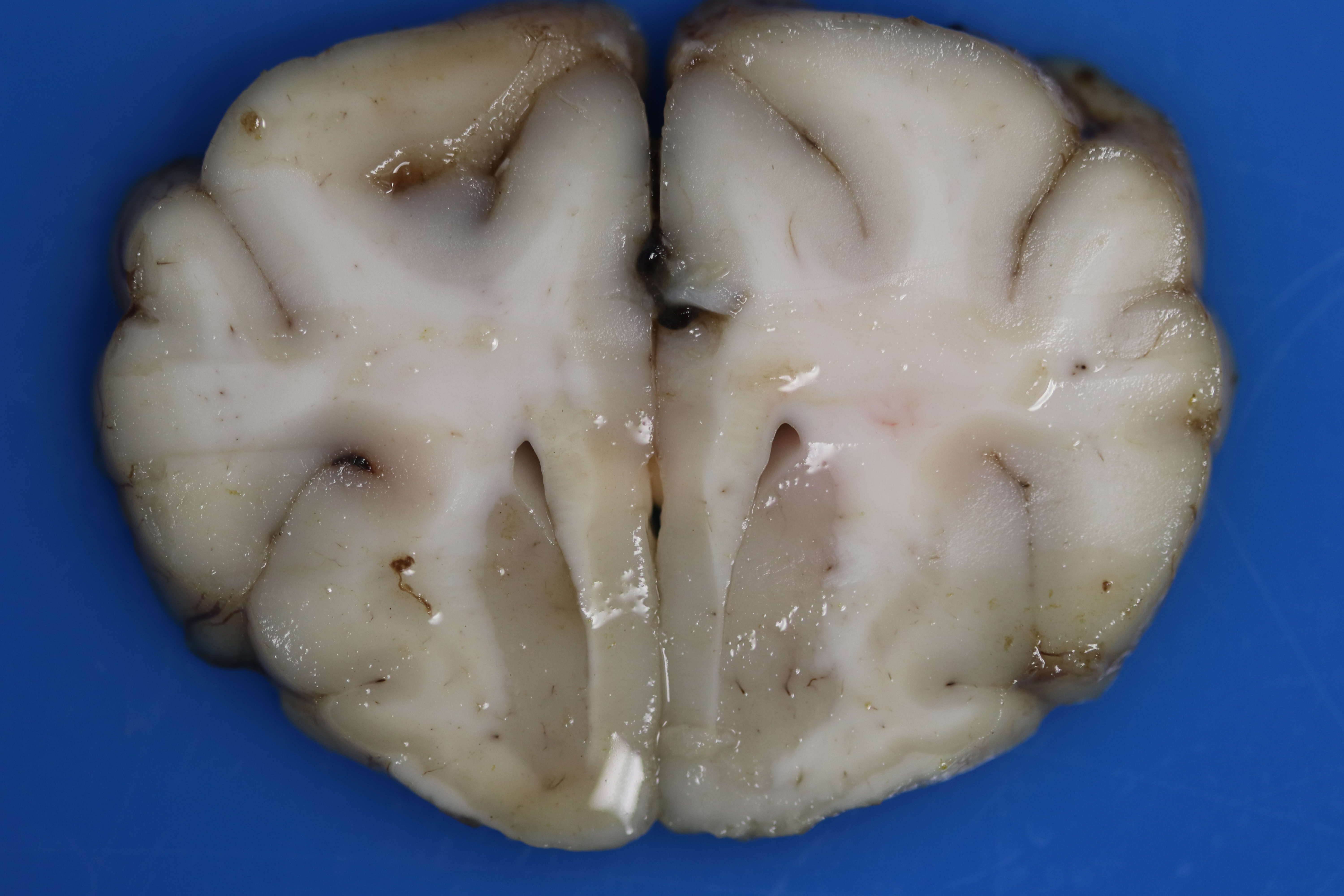
There is multifocal loss of grey-to-white matter distinction. Randomly the white matter contains poorly demarcated, soft, tan regions/streaks (tinctorial change; decreased parenchymal whiteness), which is more evident in the rostral cerebrum. Throughout the cerebral cortex, the grey and white matter there are dozens of poorly demarcated, soft, light brown-yellow foci. The meninges over the spinal cord are mildly to markedly thickened, mottled tan, brown, and dark red (hemorrhages).
Laboratory Results:
Touch imprints of the meningeal surface are of good cellular and stain quality. Sample is comprised of rafts of cohesive mildly pleomorphic epithelial cells mixed with small numbers of macrophages, lymphocytes, and plasma cells on a hemodiluted background.
Microscopic Description:
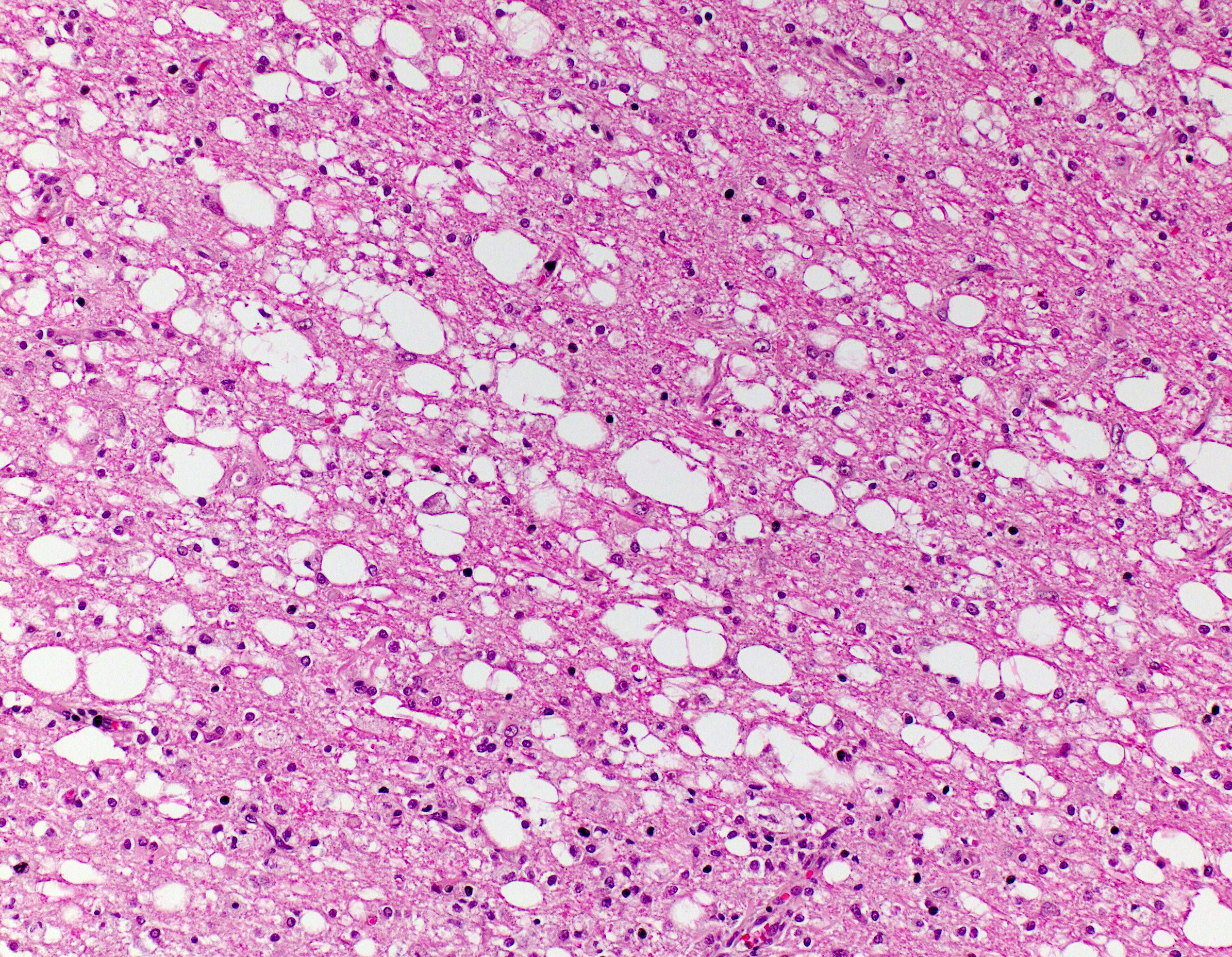
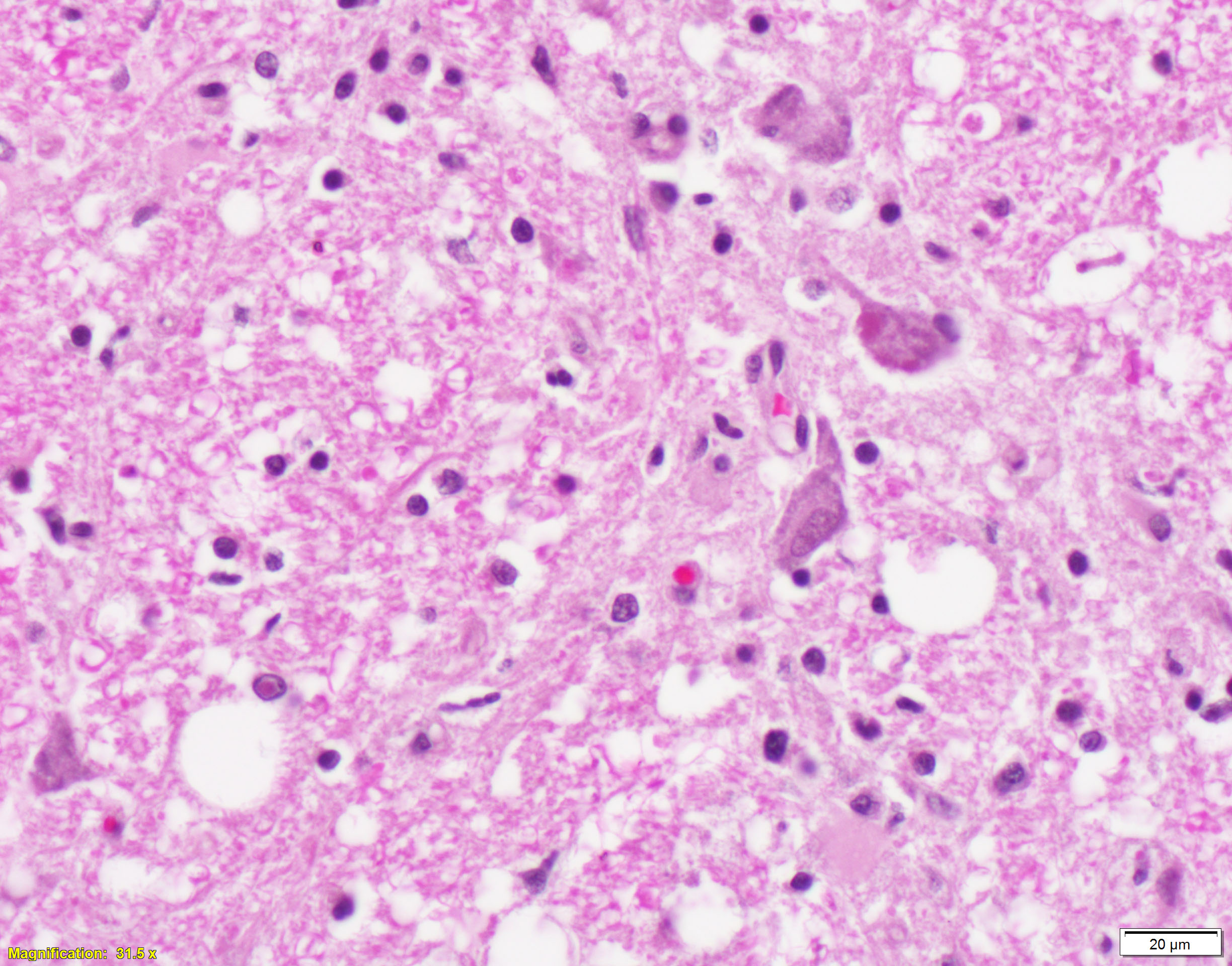
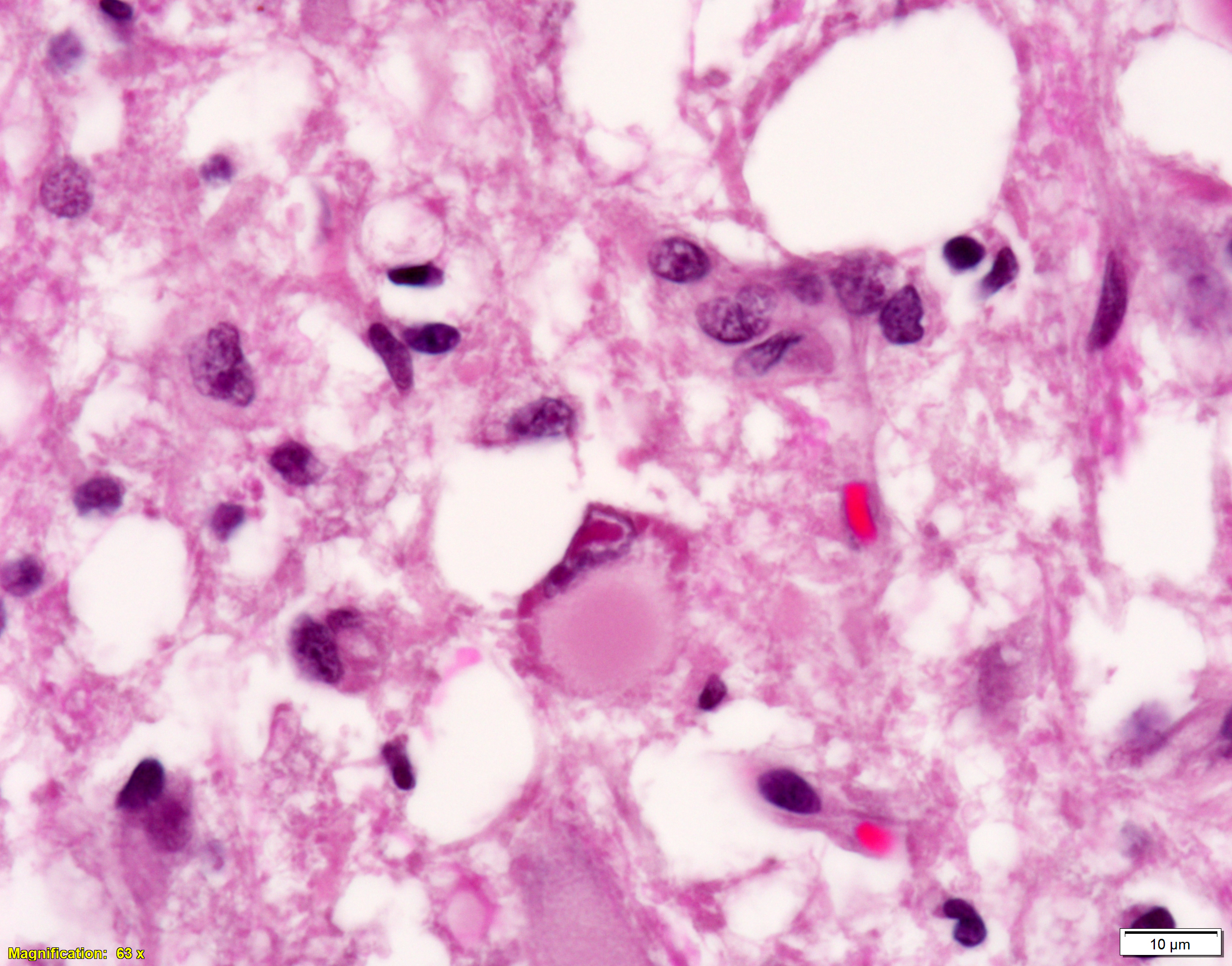
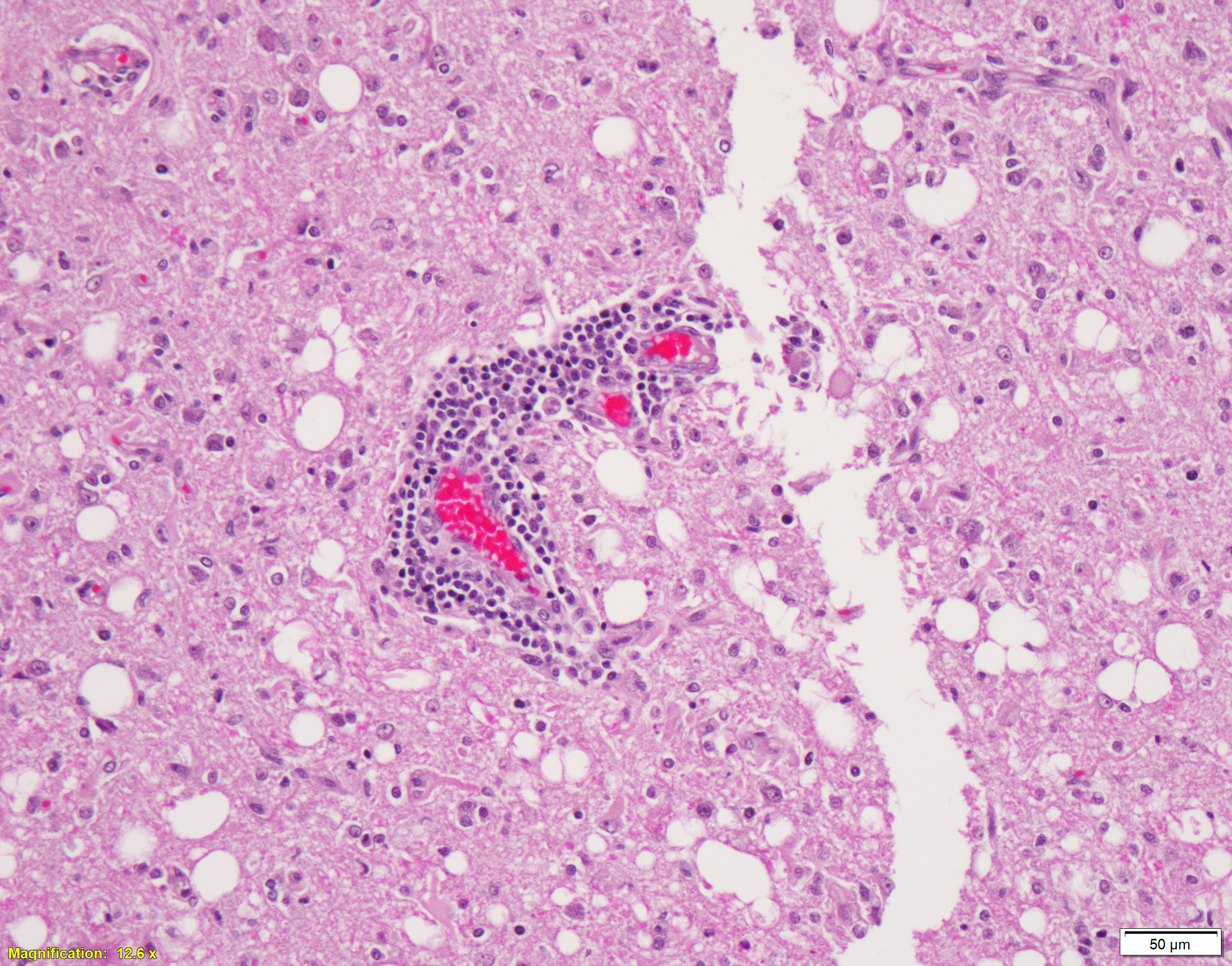
Brain, cerebral cortex -or- metencephalon at the level of the cerebellar peduncles: Subcortical white matter tracts, white matter tracts of the pons, and interspersed white matter within metencephalon are multifocally pale and vacuolated (spongiosis). Within these regions, there is myelin sheath loss, severely dilated myelin sheaths that infrequently contain macrophages or fragmented myelin (digestion chambers), and multifocal swollen axons (spheroids). Affected white matter tracts contain moderately increased numbers of reactive astrocytes. Multiple, inconsistently coalescing, poorly demarcated regions of grey matter display mild neuropil rarefaction and contain moderately to severely increased numbers of activated, reactive glial cells and gemistocytic astrocytes and few infiltrating lymphocytes and plasma cells. Astrocytes are commonly hypertrophied. Regional neurons are degenerate, multifocally display central chromatolysis, and are less frequently are shrunken, angulated, and hypereosinophilic (necrotic neurons). Neurons and astrocytes often contain intranuclear and/or -cytoplasmic 3-7µm, round to oval, hyaline, pink or magenta inclusion bodies that in nuclei are associated with chromatin margination and clearing. There are rare syncytial cells. Regional cerebral blood vessels are occasionally tortuous and are often lined by swollen, hypertrophied endothelial cells. Multifocally, the meninges are mildly infiltrated by lymphocytes, plasma cells, and macrophages. Following along Virchow-Robbin spaces and surrounding multiple cerebral blood vessels are moderate cuffs of lymphocytes, plasma cells, and lesser macrophages.
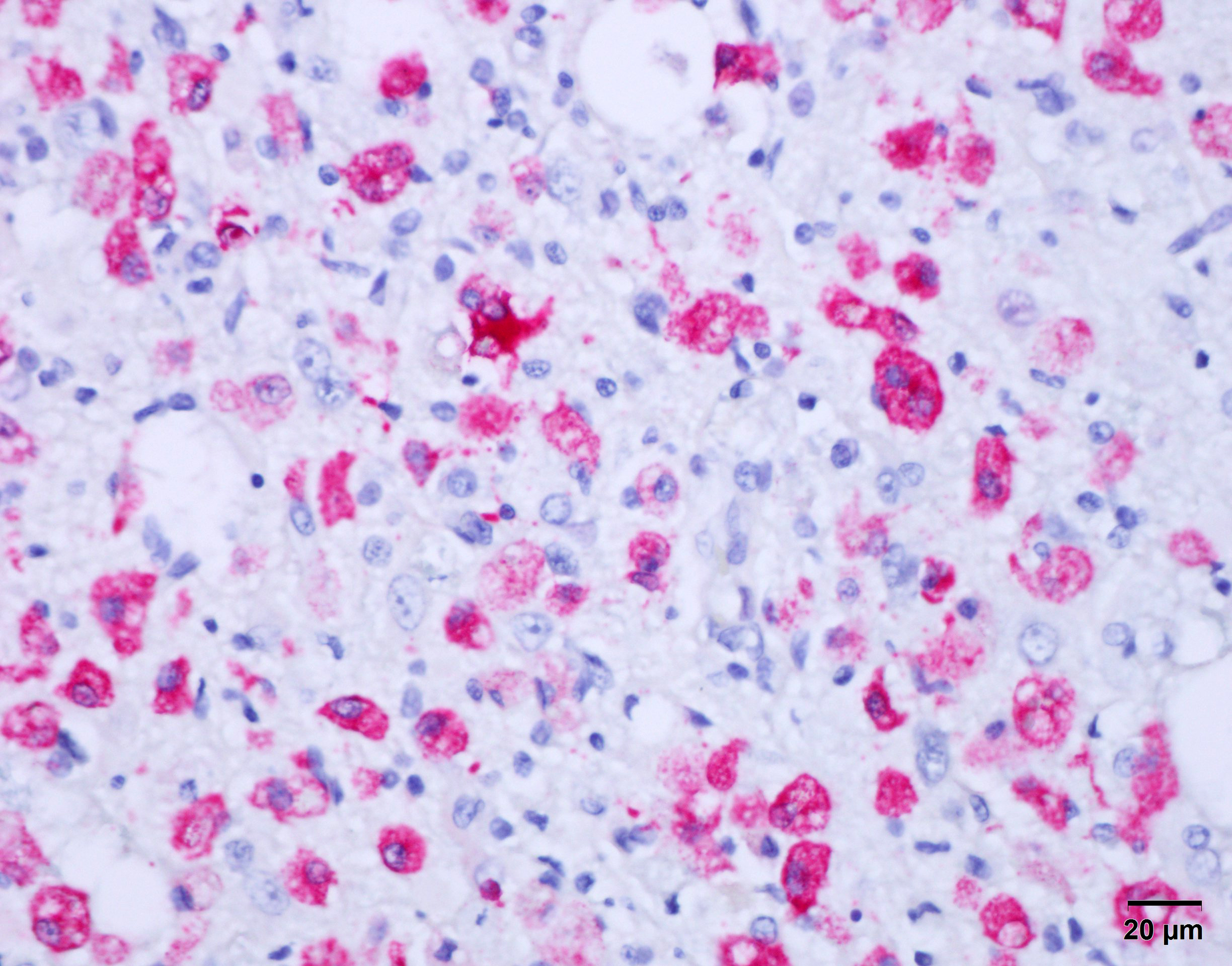
Immunohistochemical stain for canine distemper virus (Animal Health Diagnostic Center/Cornell University) was placed on sections of the brain and revealed strong cytoplasmic immunoreactivity within neurons and astrocytes and highlights axonal projections.
Contributor’s Morphologic Diagnoses:
- Demyelination and axonal degeneration, multifocal to locally extensive, moderate to marked with spongiosis, swollen myelin sheaths with fragmented myelin and macrophages (digestion chambers), and multifocal distended axons (spheroids)
- Leukoencephalitis, lymphoplasmacytic, histiocytic, chronic, multifocal, moderate with regional moderate to marked gliosis, neuronal degeneration and necrosis with mild neuronophagia, numerous intranuclear and -cytoplasmic inclusions, rare syncytial cells, and moderate lymphoplasmacytic perivascular cuffs
Contributor’s Comment:
Histologic features in this case were consistent with a demyelinating leukoencephalitis. Other findings in this dog were a single Dirofilaria immitis adult in the pulmonary artery with expected reactive changes in the blood vessel and a single metazoan parasite larva in one kidney. In dogs, differentials for leukoencephalopathy include viral (rabies, CDV, canine herpes virus) and parasitic (Neospora caninum) infections, as well as non-infectious etiologies (e.g., polyneuritis, allergic encephalitis, necrotizing encephalitis).17 Profound demyelination and the presence of both intracytoplasmic and -nuclear inclusion bodies were suggestive of paramyxovirus infection. Lesions were most prevalent and severe in the cerebral cortex and the caudal-most focus was in the metencephalon at the level cerebellar peduncles. The cerebellum, caudal brainstem, and spinal cord were spared. Immunohistochemistry for CDV confirmed intense immunoreactivity in neurons and astrocytes. Based on this patient’s signalment and history, progressive neurologic disease over two months, and histologic lesion distribution, the current case was most consistent with “old dog encephalitis” (ODE). Like other reported cases of ODE, this patient was up-to date on vaccinations.
Canine distemper virus is a highly contagious disease of carnivores with a worldwide distribution.1-10,12-13,16-23 Infections and outbreaks have been reported in numerous families within the order Carnivora, including Canidae (fox, wolves), Mustelidae (ferret, mink), Hyaenidae (hyena), Procyonidae (raccoon), Ailuridae (red panda), Ursidae (bear), Viverridae (civet, mongoose), Mephitidae (skunks), Odobenidae (walrus), Otariidae, and Felidae.3,5,9,10,12,14,21,23 Most infections involve carnivores, but infections have been reported in non-carnivore species in the orders Rodentia, Primates, Artiodactyla (pigs, cervids), Pilosa (tamandua and sloth), and Proboscidea (elephants).5,9,10,12,19 Outbreaks have been described in multiple wildlife species and hence CDV is a threat to conservation efforts worldwide.5,11,12 CDV can circulate in wildlife species even when infection rates are low in domestic dogs, suggesting that the virus persists in a complex reservoir system11 and thus both asymptomatic domestic and wildlife reservoirs play an important role in driving epidemics.5 In one review, the majority of cases outside of domestic dogs were reported in captive animals, suggesting that conditions of captivity (e.g., housing multiple mammalian species in close proximity) is a risk factor for infection.11 CDV should be a considered a differential diagnosis of disease and extinction events, even in species outside of the order Carnivora.11
Canine distemper virus (CDV) belongs to the family Paramyxoviridae within the morbillivirus genus.1-3,5-10,12,14,16,18,19,22,23 Other important morbilliviruses in veterinary medicine include Rinderpest Virus, Peste des Petits Ruminants Virus, Phocine Distemper Virus, Cetacean Morbillivirus, and Equine Morbilivirus.8-10,12,18,21,23 Important veterinary pathogens within the larger Paramyxoviridae family include those of the genera henipavirus (Hendra and Nipah Viruses), avulavirus (Newcastle Disease Virus and other avian paramyxoviruses), rubulavirus (Porcine Rublavirus, mumps), respirovirus (Sendai virus, Bovine Parainfluenza Virus 3), Metapneumovirus (Avian Rhinotracheitis Virus), ferlavirus (Fer-de-lance), and aquaparamyxovirus (Atlantic Salmon Paramyxovirus), as well as several unclassified paramyxoviruses (Bottle-nosed Dolphin paramyxovirus, Salem Virus).8,9,12,13
Paramyxoviruses are large (150-300 nm), pleomorphic, enveloped viruses with a negative-sense, single-stranded RNA genome.3,5,6,8,9,12,16,19,22,23 Viral proteins include two viral envelope glycoproteins (fusion (F) and hemagglutinin (H)), a nucleocapsid (N) protein, two transcription-associated proteins (P protein and viral polymerase (L)), and a membrane (M) protein.3,5,10,11,13,14,22 Infection is initiated through binding of the viral hemagglutinin (H) protein to SLAM (signaling lymphocyte activation molecule) and nectin-4 receptors on host immune and epithelial cells, respectively.5,9,10,22 A potential 3rd host receptor, GliaR, is suggested on glial cells.5 Thus, the highly variable H glycoprotein is the main determinant of cellular and host tropism. 5,9,10,13,22 Virulence is modulated via the fusion (F) glycoprotein, which mediates union between the virus and host plasma membranes allowing for viral penetration.5,22 Characteristic syncytial cells due to host cell fusion is likewise facilitated by the F glycoprotein.9,22 Replication occurs in the host cell cytoplasm and virions are released by budding from the plasma membrane through interactions with the membrane (M) protein.5,12,13 Infection is cytolytic with the virus killing host cells during replication and release.12,23 Virus is shed primarily in respiratory secretions, but other body secretions and fluids are infectious to a lesser extent.3,5,7 The primary modes of transmission include close contact and aerosol.3,5-7,9,10,12,22,23 As this virus is relatively labile in the environment,9,12 fomite transmission is less frequent.3, 5-7,9,10,12,22,23
After inoculation, CDV first colonizes the upper respiratory tract mucosa, where it infects local lymphocytes and is phagocytized by local macrophages and dendritic cells.6,9,12,14,21-23 Infected immune cells then travel to the regional lymph nodes and tonsils, where viral replication occurs resulting in lymphoid necrosis and depletion.3,6,9,12,15,21-23 Primary viremia results allowing for dissemination through the hemolymphatic system. 3,6,9,12,15,21-23 The resulting immunosuppression facilitates the development of opportunistic infections, 3,6,9,12,15,21-23 and CDV-associated thymic atrophy further drives immunosuppression.3,18 Secondary viremia develops through leukocyte and platelet trafficking and cell free viremia.5,6,9,12,15,21-23 The extent of viral dissemination and thus clinical disease depends on the rapidity and effectiveness of the individual’s immune response against the specific viral strain.3,9,12,13,14,23 Patients with an adequate immune response will go on to clear infection, while those that mount a poor to absent response display widespread systemic disease with high mortality.3,9,14,23 With an intermediate immune response, minor to absent mucosal disease and variable neurologic disease may be seen.3,9,14,23
Canine distemper virus is pantropic with a high affinity for lymphoid, epithelial, nervous, and ocular tissues.16,22,24 Severity of disease depends on the viral strain, age and immune status of the host, and rapidity of the immune response.3,6,9,12-14 Clinical disease in dogs is most common in puppies of 12-16 weeks of age when maternally derived, passive immunity wanes.3,6,9,12-14,22 In these young naive dogs, clinical disease typically manifests as a combination of CNS, respiratory, and/or gastrointestinal signs.9,14,22 Infection typically starts with fever and conjunctivitis and rapidly progresses to respiratory and gastrointestinal signs.3,9 Extra-nervous signs typically develop about a week post inoculation and include pyrexia, oculonasal discharge, pharyngitis, tonsilitis, otitis interna/labyrinthitis, cough, vomit, diarrhea, skin rash and pustules, hyperkeratosis of the nose and foot pads, enamel hypoplasia (due to ameloblast degeneration and necrosis), lower urinary tract signs, ocular disease (due to viral retinitis, non-suppurative optic neuritis, and optic nerve demyelination), and abortion.3,5,6,9,12-14,16-19,21-23 Histologic changes in the lungs include necrotizing bronchointerstitial pneumonia with type II pneumocyte hyperplasia and syncytial cell formation, pulmonary edema, and inclusion bodies in pneumocytes and alveolar macrophages.3 Enteric lesions attributable to the gastrointestinal signs are not well described, but virus is thought to infect the intestinal crypt epithelium.16 CDV less consistently causes an infectious myocarditis, which depending on chronicity can be accompanied by frank myocardial necrosis and/or fibrosis.3,21 In both experimental and natural infections growth retardation lattices can be seen in the bones, which in experimental models was related to transient impaired osteoclastic resorption of the primary spongiosis.4 In an outbreak in Linnaeus’s 2-toed sloth (Choloepus didactylus), marked hepatic tropism was an atypical striking feature.19 Affected dogs may die at this stage, recover fully, or go on to develop neurologic signs 1-6 weeks later.3,6,9,12,23
CDV is associated with several distinct nervous system manifestations in dogs1 that include: 1) canine distemper encephalomyelitis in immature dogs, 2) old dog encephalitis, 3) multifocal distemper encephalomyelitis in mature animals, and 3) post-vaccinal encephalitis.1,7 The latter three are uncommon manifestations confined to the CNS only.1,2,7 The syndrome that develops is directly dependent on the viral strain, host age and immune status, and neuroanatomic location of pathologic lesions.7 The most common CNS manifestation is canine distemper encephalomyelitis in immature dogs that is part of the above-described pantropic infection. 1, 2, 7,14 In these naive animals, neurologic disease can occur with or without preceding or concurrent gastrointestinal and/or respiratory signs.9,14,18,22,23 Approximately 30% of dogs infected with systemic disease ultimately develop neurologic dysfunction observed 1-6 weeks after the onset of initial signs.6,9,22,23 Hematogenous spread to the CNS allows for initial infection of and viral replication in vascular pericytes and astrocyte foot processes, endothelial cells, microglia, and choroid plexus epithelium.14,22,23 Involvement of the choroid plexus epithelium and ependyma allows for the virus to be shed into and disseminated by the CSF.3,22,23 With dissemination, virtually all cells of the CNS are susceptible to infection.3,12,14,22-23 Neurologic signs include cerebellar or vestibular ataxia, “gum chewing”, paresis to paralysis, myoclonus, tremors, circling, hyperesthesia, and epileptic seizures. 3, 5, 7, 10, 12, 17, 20-22 In a recent single case report, CDV infection presented as focal lower motor neuron signs and hyperesthesia to one forelimb, which rapidly progressed to diffuse lower motor signs of all limbs and seizures.7 This unique presentation was related to lesion distribution to the C5 and lumbar spinal cord segments.7 The main histologic lesion in naïve patients is a demyelinating leukoencephalopathy related to viral-induced injury to the neurons and olidodendroglia.10,14,17,18,21,22Acute lesions in the CNS are not overwhelmingly inflammatory and are likely related to metabolic dysfunction, decreased myelin synthesis by damaged oligodendrocytes, and microglia activation.9 With lesion progression CDV-specific immune responses and viral persistence induce an inflammatory reaction.9 Viral-induced damage to oligodendrocytes leads to highly characteristic, primary demyelination predominantly distributed to the cerebellum, periventricular white matter, spinal cord, and optic tracts. 3,6,9,14,18,20,22 Additional white matter changes depend on chronicity and include intra-myelinic edema, vascular proliferation with hypertrophied endothelial cells, infiltrating macrophages associated with myelin debris, white matter necrosis, gliosis.23,9,18,22,23 Alterations to the grey matter target the cerebral cortex, cerebellum, brainstem, and spinal cord and include non-suppurative encephalitis with perivascular cuffs.3,18,20,22 Neuronal injury is demonstrated by central chromatolysis and degenerative neurons that progress to eventual necrosis with inconsistent neuronophagia. 3,18,20,22 Intracytoplasmic and -nuclear inclusions are seen in neurons and astrocytes.12,18,23 Sclerotic astrocyte foci and myelin loss may be seen in survivors.3,12,22,23
This patient’s signalment and history (i.e., middle-age, progressive neurologic disease over two months, and up-to-date vaccination status) and pathologic findings (i.e., lack of findings consistent with systemic CDV infection, histomorphologic features confined to the CNS, and lesion distribution) were most consistent with “old dog encephalitis”. Old dog encephalitis (ODE) is a rare, unique, invariably fatal CNS-only presentation in older dogs.1-3, 9,10,12,14,18,23 A diagnosis of ODE necessitates a combination of clinical history and findings, typical histologic lesions and distribution, and absence of CDV-induced lesions in other organs.7 Affected animals are typically completely vaccinated with no evidence of previous systemic CDV infection.1,7,9,13,22 Clinical disease is insidious characterized by chronic, progressive cortical disease with motor and behavioral disturbances (i.e., circling, swaying, weaving, compulsive walking, postural reaction deficits, head pressing, and pushing on fixed objects).1-3,7,12,23 In one experimentally-induced ODE case, the dog exhibited multiple episodes of relapsing cortical and subcortical signs and epileptic seizures were part of this dog’s disease.1 Gross lesions are non-specific with ODE and include dilation of the ventricles, loss of grey-white matter distinction, and grey-brown regions in the forebrain.2,7 Histologic changes are confined to the cerebral cortex, thalamus, and mid-brain, with sparing of the caudal brainstem and spinal cord.1, 2,7,18,23 Histomorphologic features include demyelination; vacuolization of the subcortical white matter, non-suppurative encephalitis with profound perivascular cuffs; astrocytosis and astrogliosis; neuronal loss, degeneration, and necrosis; and neuropil rarefaction.1, 2,7,18,23 Inclusion bodies are numerous.1,27,18,23 Ultrastructural changes include mononuclear cuffs, viral nucleocapsids and viral budding in the grey matter, electron-dense granular material in Virchow-Robbins spaces (unclear origin), and white matter changes suggestive of abortive and inadequate axonal remyelination (i.e., naked axons surrounded by astrocyte processes and remyelinated axons with thin myelin sheaths) and chronic demyelination (i.e., loosely arranged demyelinated axons separated by inflammatory cells).1 The pathogenesis of old dog encephalitis is unclear and the host-to-virus relationship is not understood.1-3,7,9,10,12,13,18,23 Previously proposed theories have included: 1) wild-type viral persistence in a rare replication-defective state, 2) terminal result of chronic subclinical CDV encephalitis, 3) re-infection, and 4) a viral strain predisposed to persistent infection.1,2,9,12,13,23 At this time, ODE is believed to be the result of virus persistence in the CNS in a replication-defective form.1-3,7,9,10,12,13,18,23 Evidence to support this theory include: 1) ODE isolates are antigenically similar to the wild-type virus and don’t display a mutation rate beyond what is expected for an RNA virus,1,7 2) unique histomorphologic features of ODE that are less consistent with progression of previous infection (i.e., profound angiogenic distribution and atypical lesion localization),1,7 3) difficult post-infection isolation of the inoculating virus (i.e., prolonged culture and coculture with susceptible Vero cell monolayer),1 and 4) patient histories.1-3, 7,9,10,12,13,18,23 In addition, ODE has some similarities to subacute, sclerosing panencephalitis (SSPE) seen in children with persistent infections with a replication-defective measles virus.1,12,13 SSPE is slightly different from ODE in that SSPE isolates are constitutively replication defective and therefore never produce viral particles, where in ODE virions can be isolated but with significant difficulty.1,13
Other CNS-only manifestations of CDV are similarly uncommon. Multifocal distemper encephalitis in the mature dog is a rare, chronic progressive disease due to infection in middle-aged to senior dogs (4-8-years-old).2 Clinical signs include slowly progressive head tremors, hindlimb paresis, and incoordination.2 Seizures are not typical.2 Histologic changes are restricted to the CNS, where lesions are predominantly distributed to the cerebellum and spinal cord, with sparing of the cerebral cortex.2 Histologic features include a demyelinating leukoencephalitis, multifocal necrotizing, non-suppurative encephalitis, and rare intranuclear inclusion bodies.2 Post-vaccinal distemper is uncommon and presents as severe, often lethal neurologic decompensation with aggressive behavior in patient who have been recently vaccinated (within 3 weeks) with an attenuated viral strain.2,17 Histologic lesions are not well-characterized, but grey matter changes are similar to those described in natural disease with relative sparing of the white matter.2
Contributing Institution:
Animal Medical Center
510 E. 62nd Street
New York, NY 10065
JPC Diagnosis: Cerebrum: Encephalitis, lymphohistiocytic, chronic, multifocal, moderate, with spongiosis, gliosis, and neuronal and glial intranuclear and intracytoplasmic viral inclusions.
JPC Comment: The contributor provides an excellent and thorough review of systemic and neurologic manifestations of canine distemper virus infection. This week’s moderators, Drs. Eric and Juliana Lee, selected two cases of canine distemper (see Case 2 for CDV-pneumonia in a dog) to demonstrate the spectrum of disease caused by this pancytotropic virus.
CDV is closely related to two other morbilliviruses: rinderpest virus (RPV) and human measles virus (HMV). Rinderpest virus, which was recently eradicated, is the oldest of these viruses, with origins in Asia over three thousand years ago.11 The virus then spread to the Near East and Europe, and texts from Greece and Rome document rinderpest outbreaks in the fourth century BCE.15 Human measles virus was first documented around the ninth century CE, and the term morbilli, a diminutive for “morbus” or illness, was coined to differentiate it as a “small” illness compared to smallpox.11 It is thought that human measles virus originated from RPV or a common morbillivirus precursor of ungulates and phylogenetic analysis suggests that it diverged around 12th century CE.11,15 Canine distemper virus is much younger than both RPV and HMV, with the first reports in Central and South America in the early 1700s.11 Two decades years after its first appearance in the New World, CDV was reported in Europe and its initial epizootic spread had a high initial rate of mortality, characteristic of a newly introduced pathogen.15
Several hypotheses have been proposed regarding the evolution and first occurrence of CDV in the Americas, including a sylvatic virus that jumped to domestic dogs or a virus of domestic dogs belonging to Paleoindians which spread with their movement.15 A recent study by Uhl et al evaluated historical records, genetic and epidemiologic characteristics, and paleopathologic data to uncover the origins of CDV, and ultimately, they supported a different theory: that CDV began when HMV jumped from humans to dogs.15 First, the group evaluated 2335 permanent teeth from 96 dog skeletons from the Weyanoke Old Town (750-1470 CE) site in Virginia and found no evidence of enamel hypoplasia (indicative of in utero CDV infection); additionally, enamel lesions were absent in 42 dogs from South American site dating from 1030-1324 CE.15 These findings support the fact that CDV arose after the arrival of European explorers and the HMV they carried. Second, the remarkable genetic similarities in sequence, structure, antigenic epitopes, and host receptors they target indicate CDV and HMV likely arose from a common ancestor.15 Codon usage analysis also indicates that CDV likely evolved from a virus that previously infected humans, as its codon usage bias (which reflects evolutionary host adaptation) favors that of a human over a dog.15 Finally, the authors used historical records to demonstrate the massive social disruption and upheaval caused by European activities coupled with overwhelming illness and death with the introduction of HMV to naïve populations provided ample opportunity for the virus to jump species and adapt to domestic dogs.15 While not definitive proof, this interdisciplinary evidence supports the hypothesis that CDV infection in dogs arose from HMV epidemics in native South American populations.15
Conference participants debated whether demyelination or encephalitis were the primary lesions. Ultimately, participants felt the inflammation was more significant, and the number of gitter cells and level of myelin debris did not reach a level to support demyelination in the morphologic diagnosis. The term spongiosis was used to describe the white matter vacuolation visible from sub-gross magnification and could be the end-state of either of these two processes.
References:
- Axthelm ML and Krakowka S. Experimental Old Dog Encephalitis (ODE) in a Gnotobiotic Dog. Vet Pathol. 1998; 35: 527-534.
- Cantile C and Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 384-385.
- Caswell JL and Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 574-576.
- Craig LE, Dittmer KE, and Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 104-105.
- Duque-Valencia J, Sarute N, Olarte-Castillo XA, et al. Evolution and Interspecies Transmission of Canine Distemper Virus - An Outlook of the Diverse Evolutionary Landscape of a Multi-Host Virus. Viruses. 2019; 11: 582.
- Green L, Cook L, Martinez M, et al. Distemper Encephalomyelitis Presenting with Lower Motor Neuron Signs in a Young Dog. JAAHA. 2020; 56: 127-132.
- Headly SA, Amude AM, Alfieri AF, et al. Molecular detection of Canine Distemper Virus and the immunohistochemical characterization of the neurologic lesions of in naturally occurring Old Dog Encephalitis. JVDI. 2009; 21: 588-597.
- Maclachlan NJ and Dubovi EJ. Frenner’s Veterinary Virology. 5th Elsevier. 2017: 327-328.
- Martella V, Elia G, and Buonavoglia C. Canine Distemper Virus. Vet Clinic Small Anim. 2008; 38: 787-797.
- Martinez Gutierrez M and Ruiz-Saenz J. Diversity of Susceptible Hosts in Canine Distemper Infection: A Systemic Review and Data Synthesis. BMC Veterinary Research. 2016; 12: 78.
- Nambuli S, Sharp CR, Acciardo AS, Drexler JF, Duprex WP. Mapping the evolutionary trajectories of morbilliviruses: what, where, and wither. Curr Opin Virology. 2016; 16:95-105.
- Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC. Veterinary Microbiology and Microbial Disease. Blackwell publishing. Ames Iowa. 2006: 381-389.
- Rima BK, Baczko K, Imagawa DT, et al. Humoral Immune Response in Dogs with Old Dog Encephalitis and Chronic Distemper Meningoencephalitis. J Gen Virol. 1987; 68: 1723-1735.
- Roody PJ, Singh K, and Basavarajappa MS. Pathology in Practice. JAVMA. 2012; 240(10): 1169-1171.
- Uhl EW, Kelderhouse C, Buikstra J, Blick JP, Bolon B, Hogan RJ. New world origin of canine distemper: Interdisciplinary insights. Int Jour Paleopathol. 2019, 24:266-278.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 116-117.
- Valli VEO, Kiupel M, Bienzle, and Wood RD. Hemopoeitic System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 207, & 145-146.
- Vandevelde M, Higgins RJ, and Oevermann A. Veterinary Neuropathology: Essentials of Theory and Practice. 1st Wiley-Blackwell. 2012: 61-3, 54, & 57.
- Watson AM, Cushing AC, Sheldon JD, et al. Natural Canine Distemper Virus Infection in Linnaeus’s 2-Toed Sloths (Choloepus didactylus). Veterinary Pathology. 2020; 57(2): 311-315.
- Wilcock BP and Njaa BL. Special Senses. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 474.
- Young Kim D, Zinn MM, Odemuyiwa SO, et al. Myocarditis caused by naturally acquired canine distemper virus infection in 4 dogs. JVDI. 2021; 33(1): 167-169.
- Zachary JF. Mechanisms of Microbial Infections. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th Elsevier Mosby. St Louis. 2012: 226-227.
- Zachary JF. Nervous System. In: Zachary JF and McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th Elsevier Mosby. St Louis. 2012: 854-855.