Signalment:
2-month-old male
crossbreed calf (
Bos
taurus).Respiratory issues
in multiple 2-month old calves.
Gross Description:
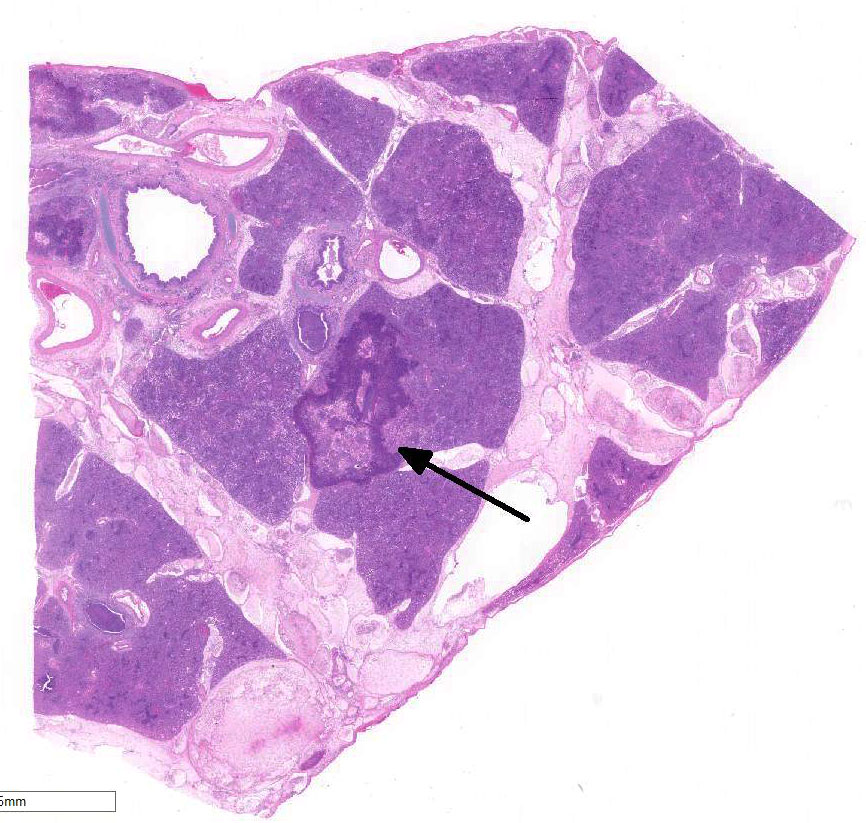
Cranioventral lung
lobes were consolidated and covered in areas with fibrin.
Histopathologic Description:
Lung:
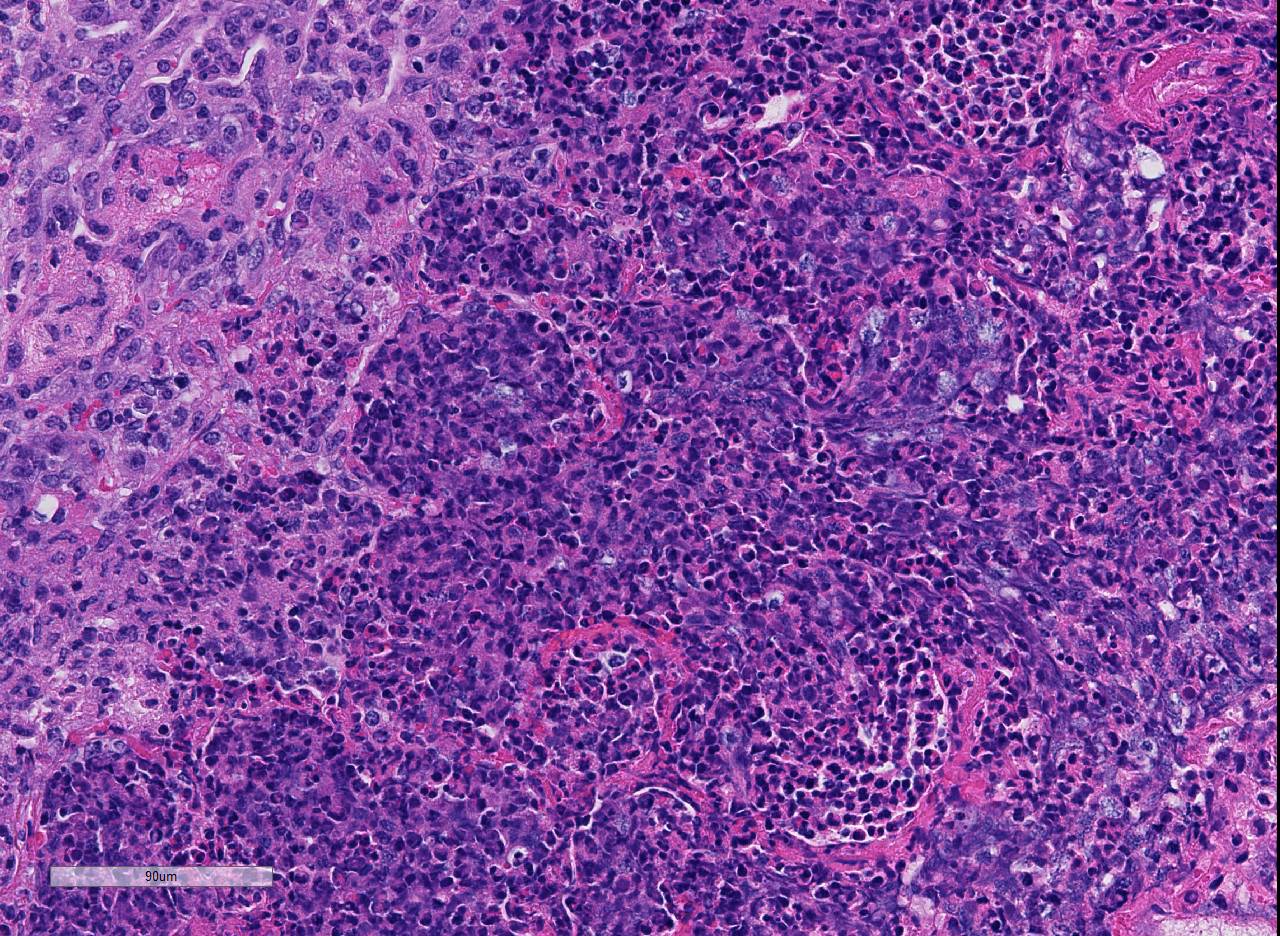
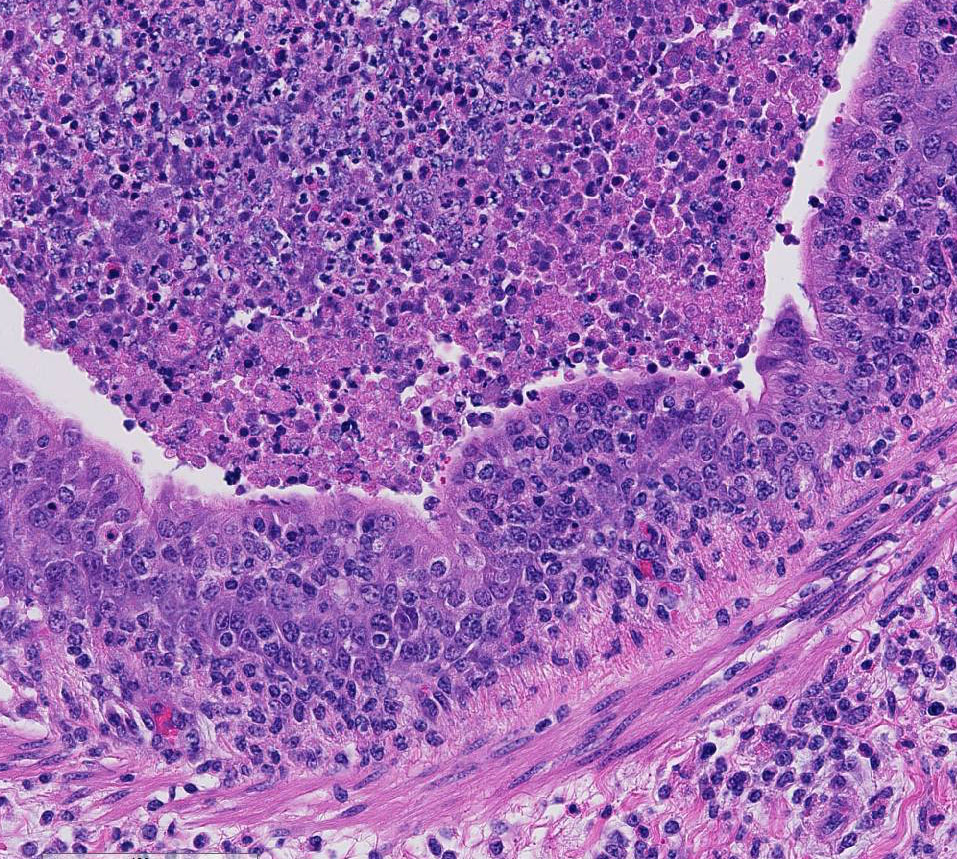
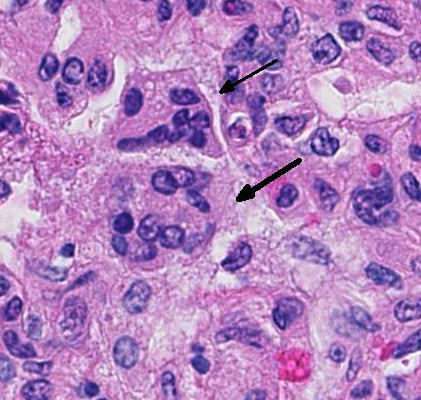
There are marked multifocal and extensive areas of necrosis demarcated by dense
zones of neutrophils which form a subgross mosaic pattern. The necrotic
regions contain large amounts of seroproteinaceous fluid, red blood cells,
neutrophils and cell debris; some cells are degenerate with elongate nuclei
(streaming leukocytes/oat cells). In remaining tissue, multifocal bronchi and
bronchioles are dilated and contain large numbers of neutrophils and cell
debris. There is diffuse thickening of the inter-lobular septa by edema and
infiltrates of neutrophils. In one lobular area there is moderate thickening
of alveolar septa by mild to moderate type II cell hyperplasia and occasional
infiltrates of lymphocytes and macrophages. The pleura is thickened by edema
and covered in some areas by a thick mat of fibrin and cell debris.
Lab Results:
Lung:
Mannheimia haemolytica
Immunohistochemistry: Bovine viral diarrhea
virus (BVD)
Condition:
Bovine Respiratory Disease Complex/Mannheimia haemolytica
Contributor Comment:
Bovine respiratory
disease (BRD) complex is multifactorial and susceptibility is related to
stress, environmental and housing conditions, management, hydration and immune
status, and exposure/levels of microbial pathogens.
5 Feedlot
density has increased progressively in the United States and global demand for
beef is trending upward; therefore, the incidence of BRD may continue to increase
over time.
5 Viral infections with agents such as bovine respiratory
syncytial virus (BRSV), bovine parainfluenza virus 3 (BPIV-3), bovine viral
diarrhea virus (BVDV), bovine coronavirus (BCV), and bovine herpesvirus (BHV-1)
can damage mucosal epithelia, decrease mucin production, as well as hinder innate
and adaptive immune responses. This predisposes calves to secondary infections
with bacteria, such as
Mannheimia haemolytica and Histophilus
somni, as in this case, as well as Pasteurella multocida, and Mycoplasma
bovis.1
In a 2005 study,
the prevalence of BVDV was determined in 2000 cattle arriving to a feedlot.
The prevalence of persistently infected (PI) cattle was only 0.3%, but 2.6% of chronically
ill cattle, and 2.5% of dead cattle were BPIV-3 positive. Cattle exposed to a
PI animal had an increased risk for treatment of BRD by 43%. In total, 15.9%
of initial respiratory tract disease conditions were associated with exposure
to a PI animal. Thus, very few cattle arrive to feedlot PI; however, those
cattle are much more likely to require treatment and at place other cattle
at-risk for incidence of respiratory disease.
4,6 Of the subtypes of
BVDV, BVDV1b subtype is more commonly identified than BVDV1a and BVDV2a based
on a survey of BVD isolates of cattle entering feedlots.
3
Experimentally, it has also been shown that exposure of steers to steers PI
with BVDV enhances
M. haemolytica disease severity.
1
In this case, the
animal had significant BVDV antigen detected by immunohistochemistry, which
could alter lung and mucosal (e.g. tonsil) immunity, thus pre-disposing to
enhanced bacterial rep-lication/colonization. Other factors such as stress and
other viruses (e.g., BRSV, BPIV-3, coronavirus, BHV-1), could have contributed
to this predisposition along with the BVD, although lesions of RSV, BPIV-3, and
BHV-1 were not seen and BHV (IBR), BCV, BRSV, and BPIV-3 were not detected by
PCR.
JPC Diagnosis:
Lung:
Bronchopneumonia, fibrinosuppurative and necrotizing, diffuse, severe, with
numerous necrotic leukocytes (oat cells)
Conference Comment:
This case is an excellent example of a classic presentation of the bovine
respiratory disease complex (BRD).
Mannheimia haemolytica is a
gram-negative coccobacillus of the
Pasteurellaceae family. It is
typically is a commensal bacterium in the nasopharynx and tonsillar crypts of
immunocompetent ruminants.
2 Many members of this family of bacteria
can produce respiratory disease and septicemia in naïve and immunosuppressed
domestic animals.
2,4,5 Other common pathogenic members of this
family include:
Pasteurella multocida of rabbits, swine, ruminants, and
cats;
Bibersteinia trehalosi and
Histophilus somni in ruminants;
Actinobacillus
pleuropneumoniae,
A. suis,
and Haemophilus parasuis in swine;
and
A. equuli in horses.
2
There are twelve
serotypes of
M. haemolytica based on their capsular polysaccharide
antigens.
2,5 Serotypes 1 and 2 are typically isolated from the upper
respiratory tract of cattle as part of the normal commensal flora;
2 however,
in a naïve or stressed animal, serotype 1 is often isolated from pneumonic lungs.
As mentioned by the contributor, stress and cold weather causes the
proliferation of commensal bacteria in the upper respiratory tract,
overwhelming pulmonary defenses. In addition, co-infection with the respiratory
viruses mentioned above can reduce mucociliary escalator clearance and impair
the function of alveolar macrophages.
2
Conference
participants briefly discussed the various virulence factors for
M.
haemolytica and how they contribute to the classic histopathologic
appearance of this case. Virulence factors include: leukotoxin,
lipopolysaccharide, capsular polysaccharide, transferring-binding proteins A
and B, O-sialogylcoprotease, neuraminidase, IgG1-specific protease, outer
membrane proteins, adhesins and fimbriae.
2 These virulence factors
allow
M. haemolytica to resist host clearance, avoid host defenses, and impair
leukocyte function. The result is massive recruitment of neutrophils into
alveoli, as seen in this case.
2,5 Neutrophils are ineffective at
killing bacteria and cause damage to capillary endothelial cells, resulting in hemorrhage,
fibrin and edema in alveolar, interstitial, and interlobular spaces.
2
Leukotoxin, a type
of repeats in toxin (RTX), is an important exotoxin produced by
M.
haemolytica. It induces leukocyte recruitment at low concentrations and
lysis of leukocytes and platelets in high concentrations.
2
Leukotoxin binds to CD18 on the leukocyte surface, forming a pore in the cell
membrane. Interestingly, BoHV-1 causes the up-regulation of CD18 on the surface
of neutrophils which makes these cells more susceptible to leukotoxin.
2
Lytic and necrotic leukocytes exhibit a streaming pattern of basophilic
chromatin and are commonly referred to as oat cells.
1,2,5,6
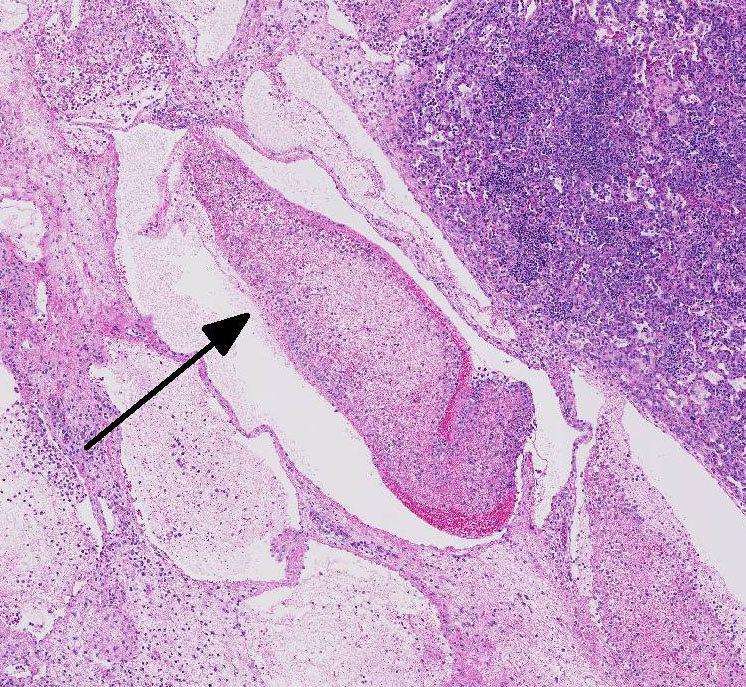
Most conference
participants noted multi-focal fibrin thrombi present throughout pulmonary
parenchyma. Additionally, in many slides, there was a sharply demarcated area
of tinctorial change with loss of differential staining surrounded by a band of
degenerate neutrophils- interpreted as an infarct. Focal areas of coagulation
necrosis and infarction are characteristic for
M. haemolytica.
2
These are secondary to thrombosis as well as direct damage to the lung by
leukocyte secretion of pro-inflammatory IL-8, oxygen radicals, and nitric oxide
synthetase. Activated alveolar macrophages also express tissue factor, which
promotes the formation of fibrin thrombi.
2
References:
1. Burciaga-Robles L, Step D, Krehbiel C, et al. Effects of
exposure to calves persistently infected with bovine viral diarrhea virus type
1b and subsequent infection with
Mannheima haemolytica on clinical signs
and immune variables: Model for bovine respiratory disease via viral and
bacterial interaction.
J An Sci. 2010; 88:2166-2178.
2. Caswell J, Williams K. Respiratory system, In: Maxie MG, ed.
Jubb,
Kennedy, and Palmers Pathology of Domestic Animals. Vol 1. 6th ed.
Philadelphia, PA: Elsevier Saunders; 2016: 537-546.
3. Fulton W, Ridpath J, Ore S, et al. Bovine viral diarrhea
virus (BVDV) subgenotypes in diagnostic laboratory accessions: Distribution of
BVDV 1a, 1b and 2a subgenotypes.
Vet Microbiol. 2005;
111:35-40.
4. Loneragan G, Thomson D, Montgomery D, et al. Prevalence,
outcome, and health consequences associated with persistent infection with
bovine viral diarrhea virus in feedlot cattle.
J Am Vet Med Assoc.
2005; 226:595-601.
5. Mosier D. Review of BRD pathogenesis: The old and the new.
Anim Health Res Rev. 2014;
15:166-168.
6. OConner A, Sorden S, Apley M. Association between the
existence of calves persistently infected with bovine virus diarrhea virus and
commingling on pen morbidity in feedlot cattle.
Am J Vet Res. 2005;
66:2130-2134.